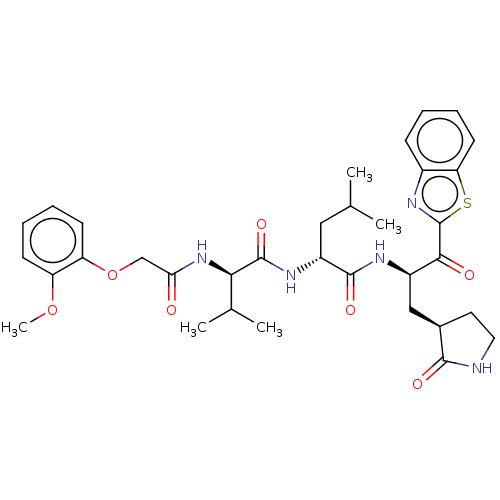
null
SMILES COc1ccccc1OCC(=O)N[C@H](C(C)C)C(=O)N[C@H](CC(C)C)C(=O)N[C@H](C[C@H]1CCNC1=O)C(=O)c1nc2ccccc2s1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 512689
Found 2 hits for monomerid = 512689
Affinity DataKi: 4.10nMAssay Description:This is a review article. Please point to the original journal.More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:This is a review article. Please point to the original journal.More data for this Ligand-Target Pair