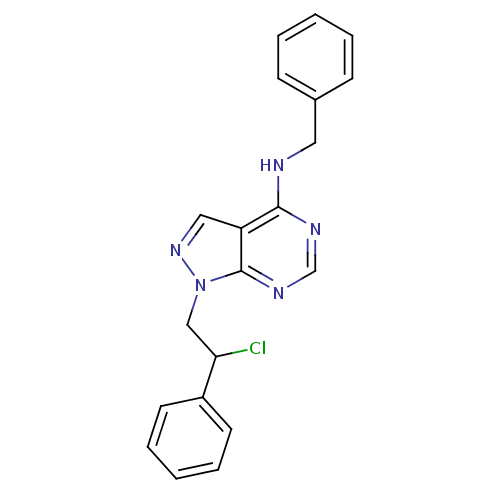
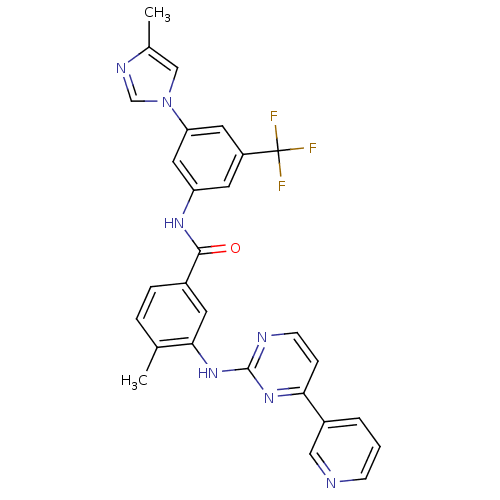
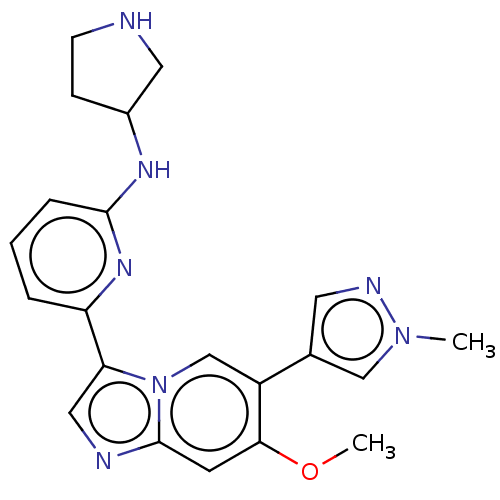
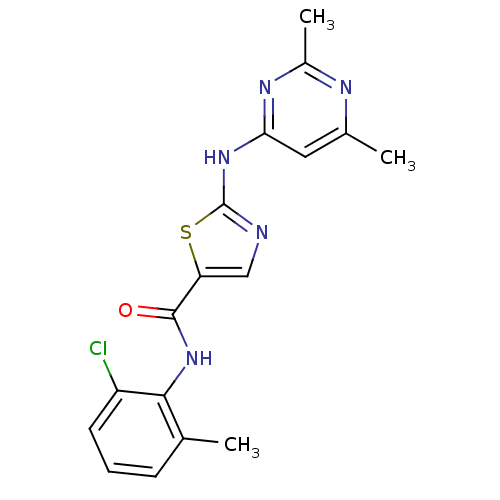
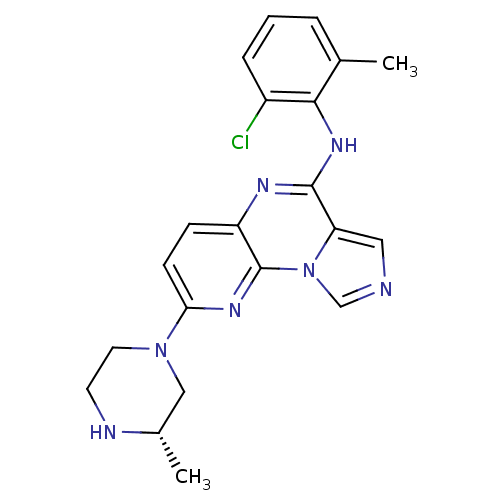
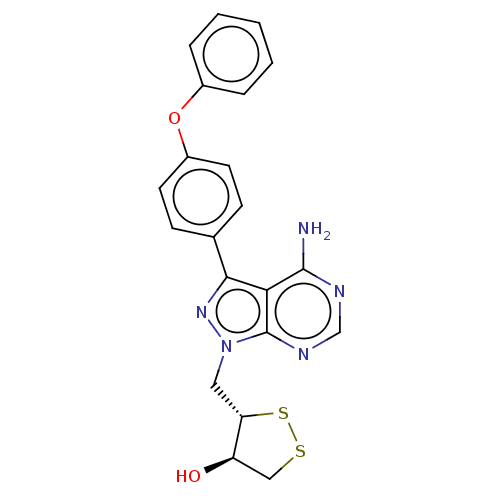
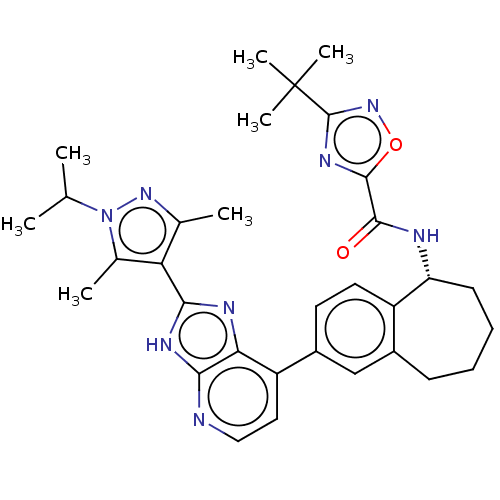
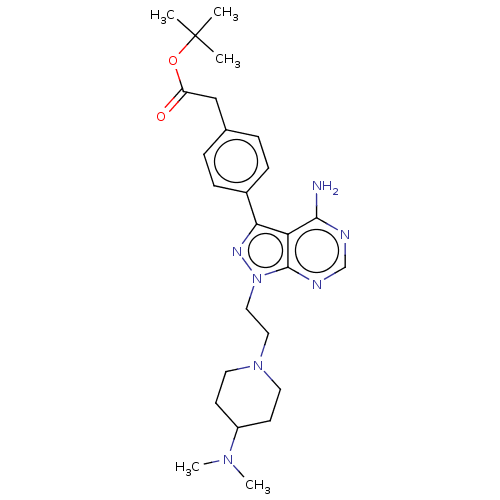
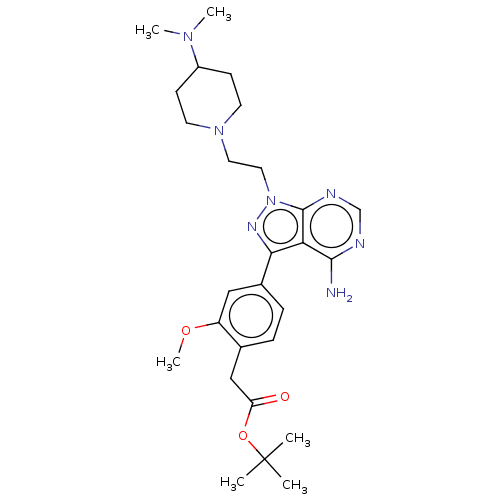
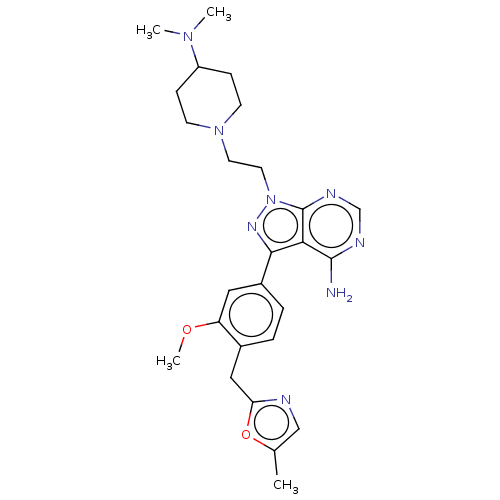
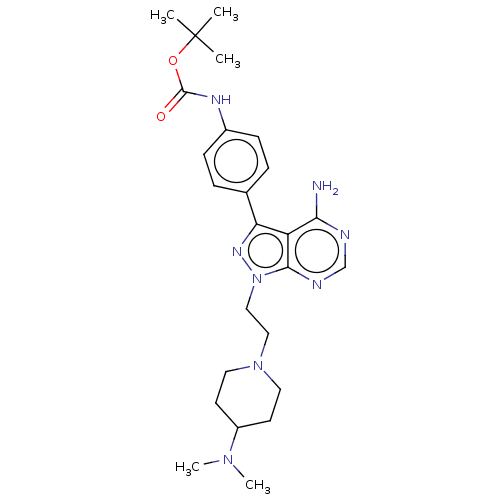
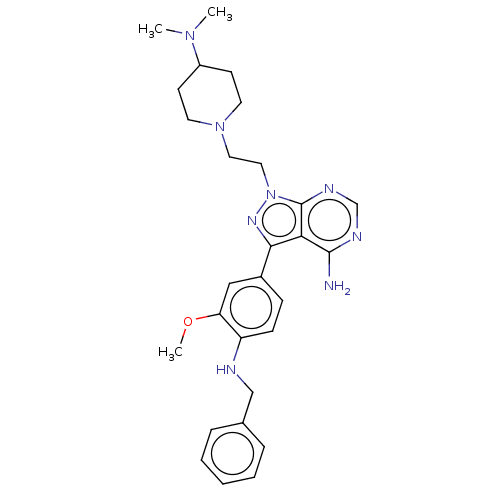
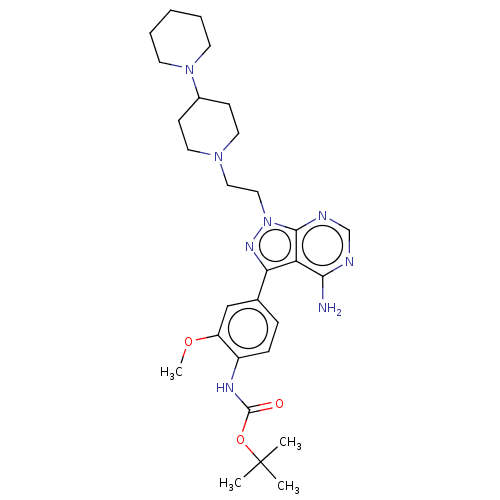
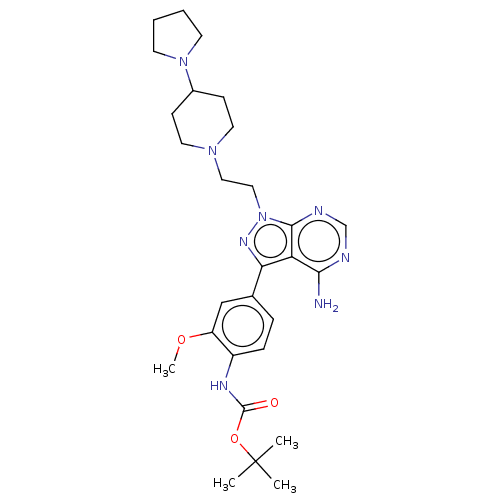
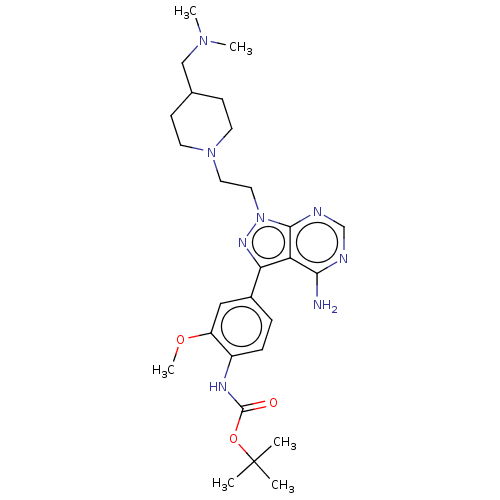
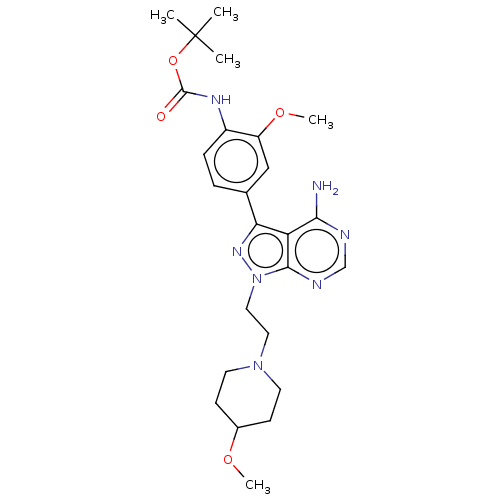
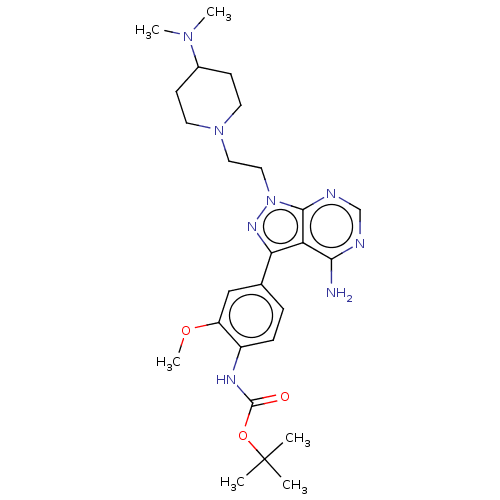
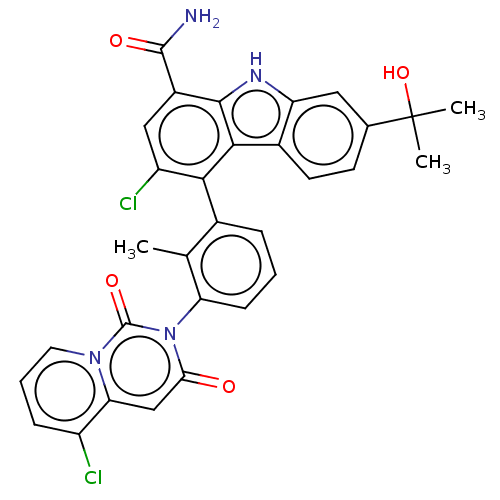
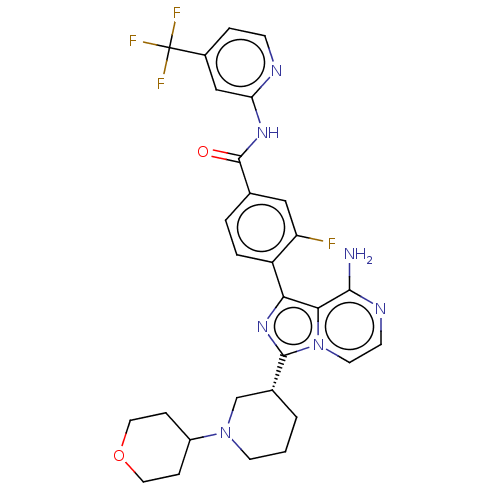
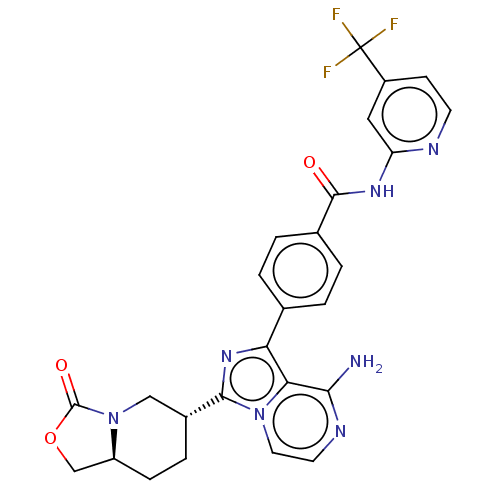
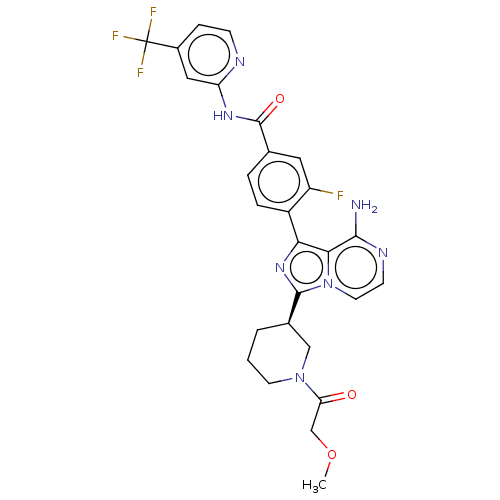
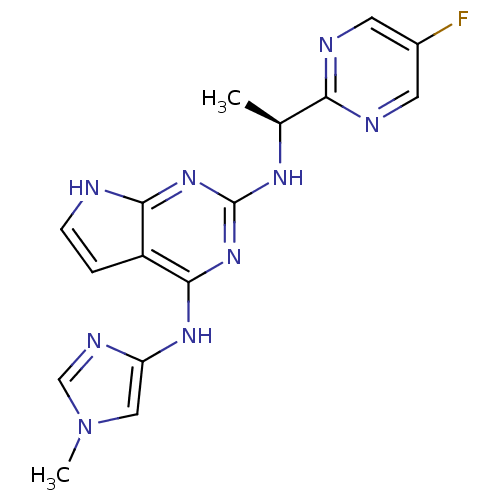
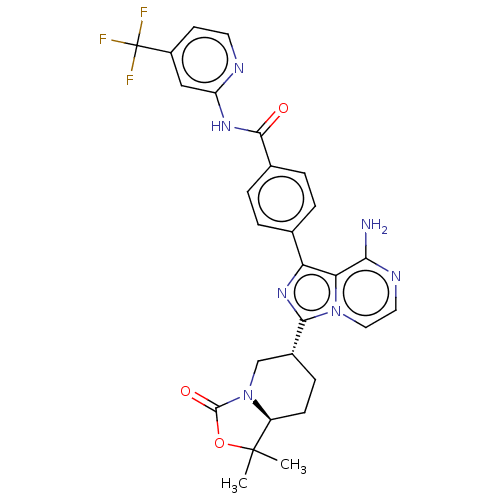
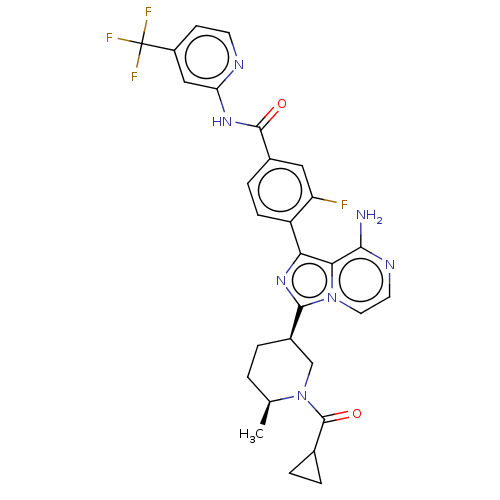
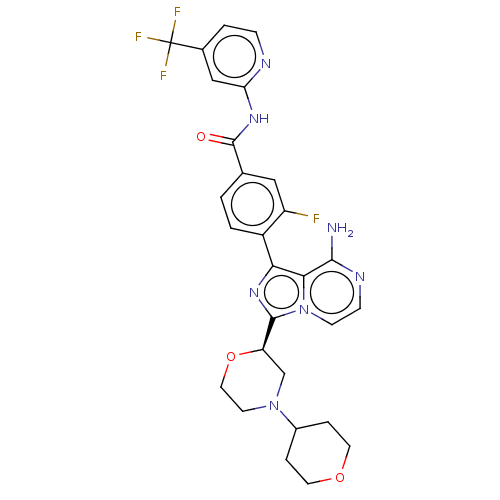
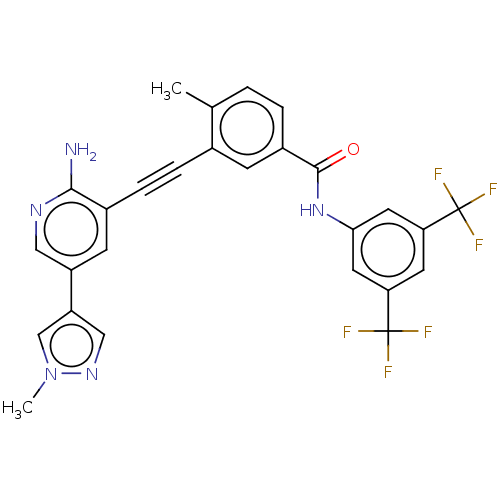
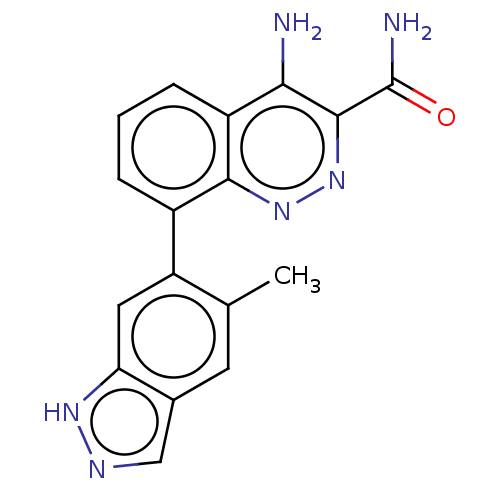
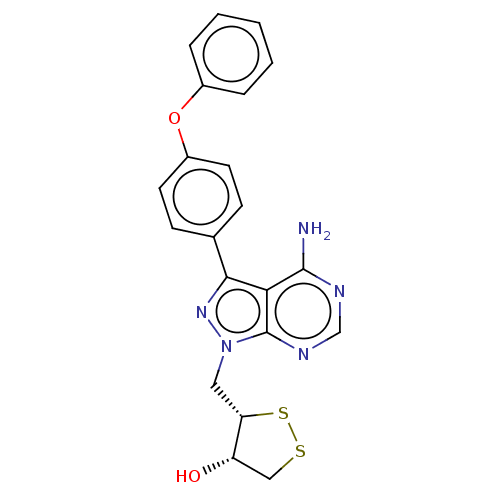
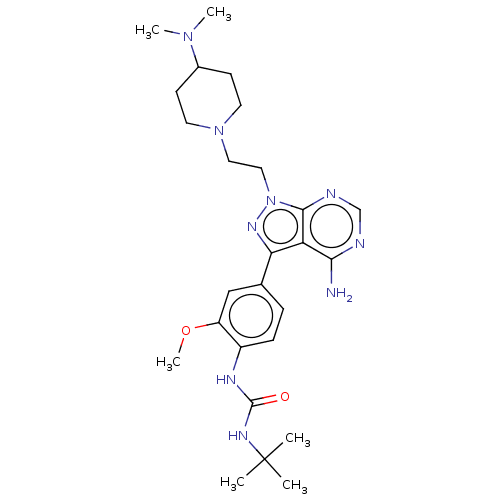
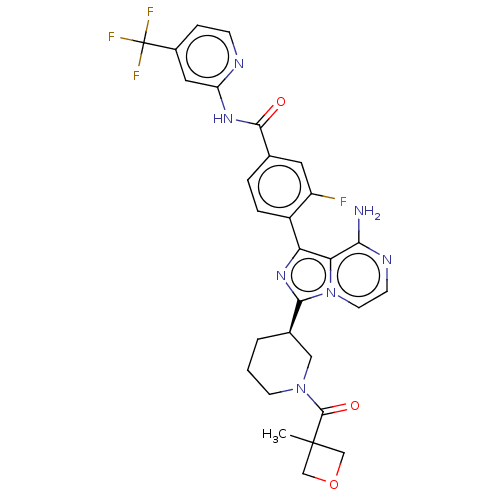
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataKi: 3.47E+3nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 0.658nMAssay Description:Inhibition of human FGR using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against Fgr kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human FGR using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human FGR using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human FGR using poly[Glu:Tyr] (4:1) as substrate preincubated for 60 mins followed by [gamma-33P]-ATP addition and measured after 120 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of Fgr protein kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 3.70nMpH: 8.0Assay Description:The inhibitory activity measurement against the kinases mentioned above was measured in the kinase inhibition reaction mixture containing 2 ul purifi...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 5.40nMAssay Description:Inhibition of human FGRMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Preparation of Compounds to be Tested:1) Using DMSO to prepare 50× compound stock solutions (same as the stock solution in Example 34) for later use;...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of human recombinant FGR using Ulight-TK peptide as substrate measured after 60 mins in the presence of ATP by LANCE methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human FgrMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human FGRMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of FGR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of human recombinant FGR (456 to 770) radiometric scintillation counting analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of human recombinant FgrMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 52nMAssay Description:IC50 Table 13-20: The protocol calls for test compound of the invention to be incubated with kinase, substrate, cofactors, and radio-isotope-labeled ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Inhibition of human FGRMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fgr(Homo sapiens (Human))
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction...More data for this Ligand-Target Pair