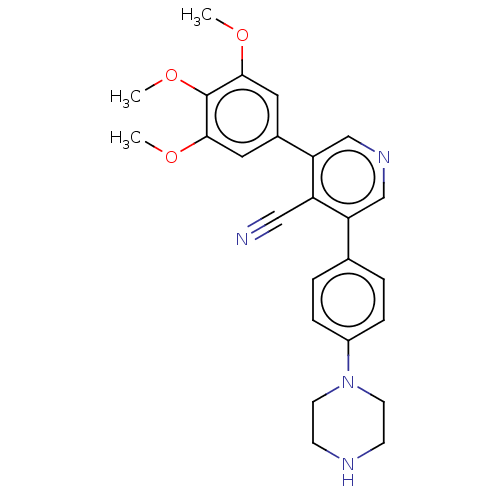
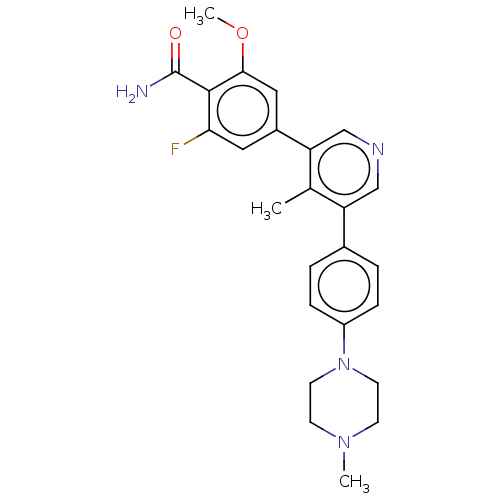
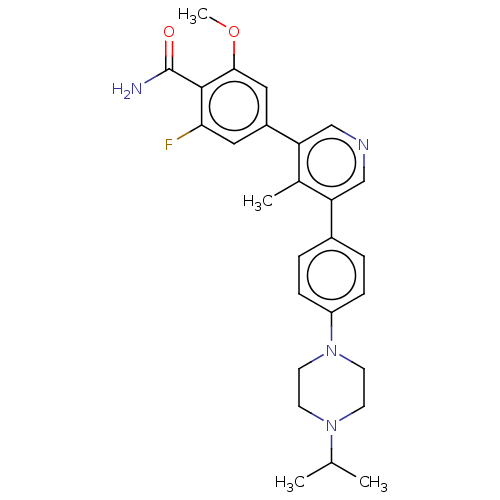
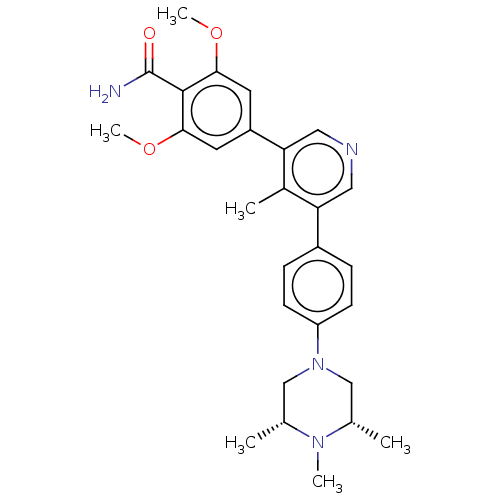
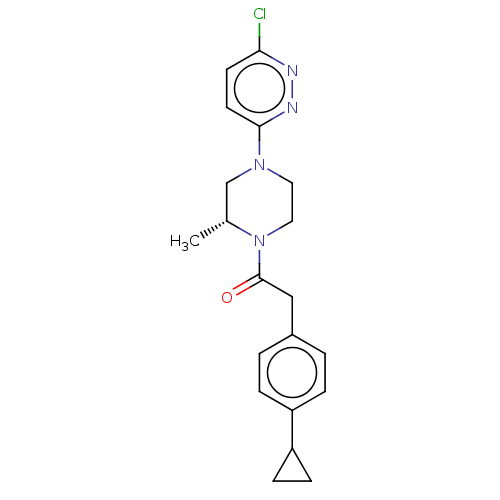
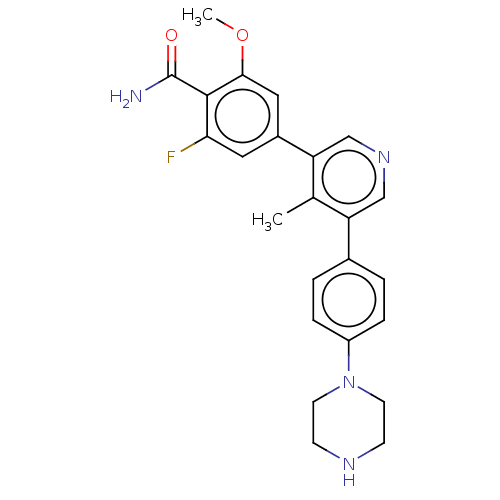
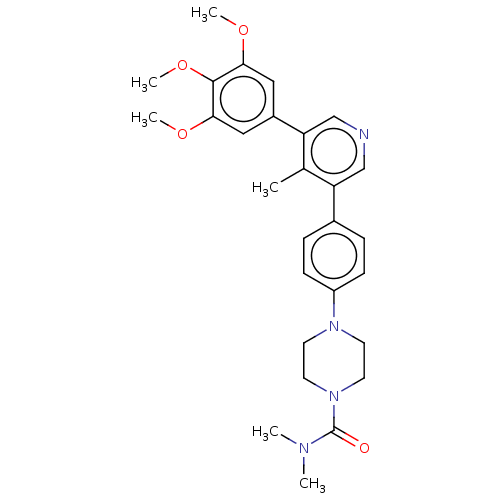
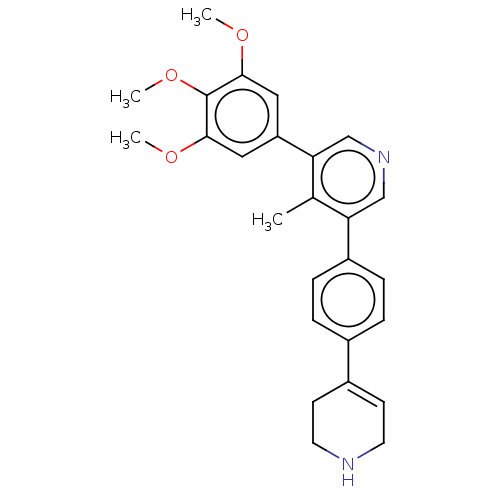
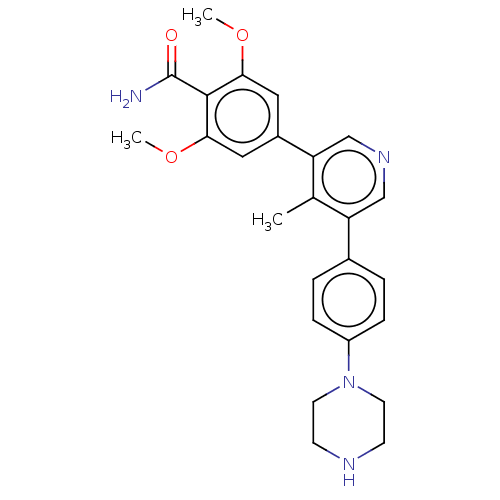
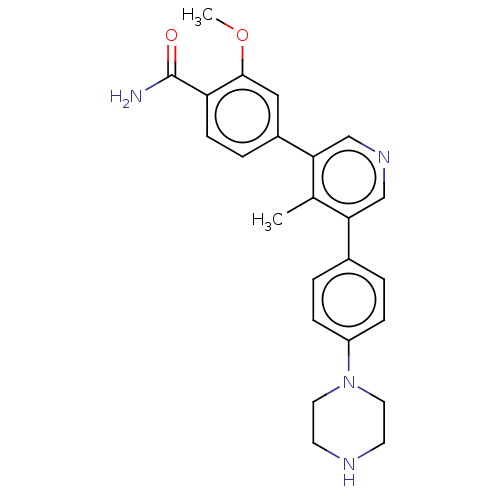
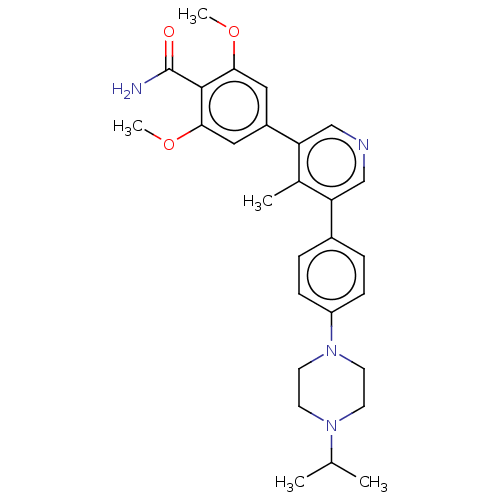
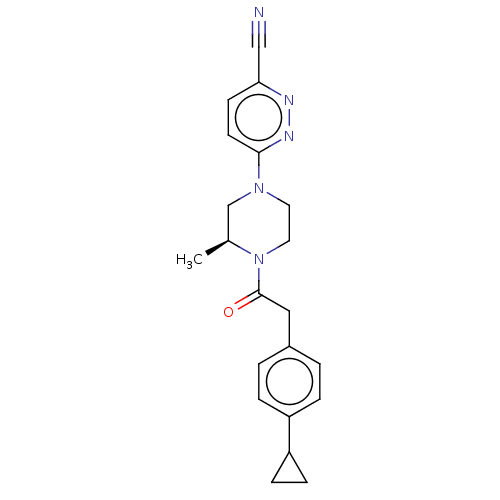
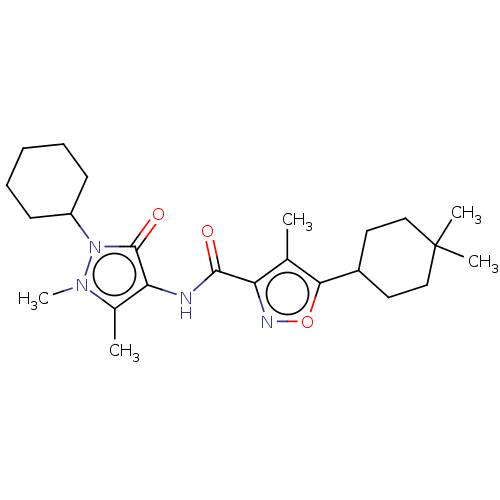
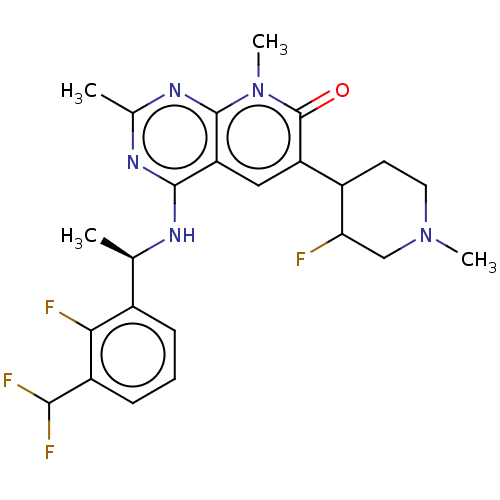
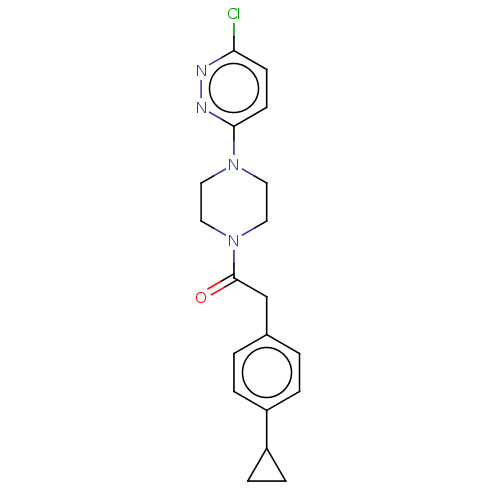
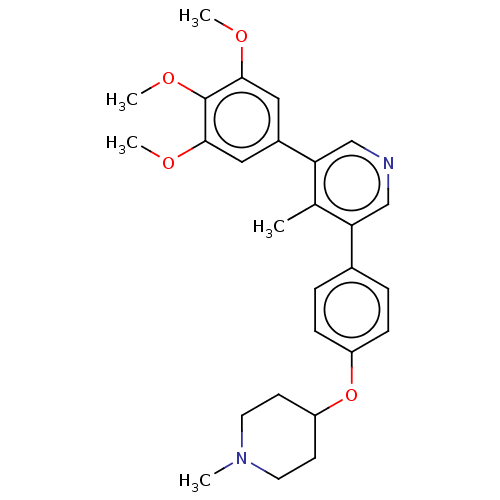
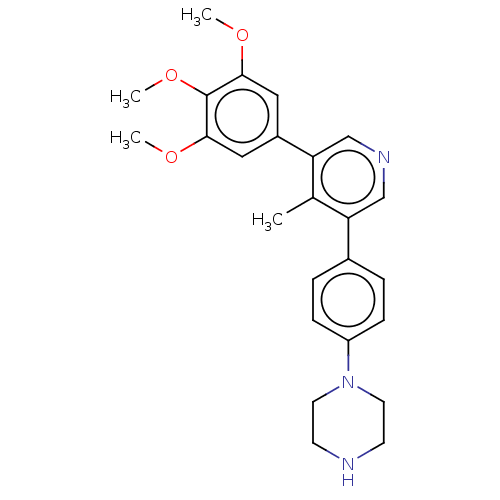
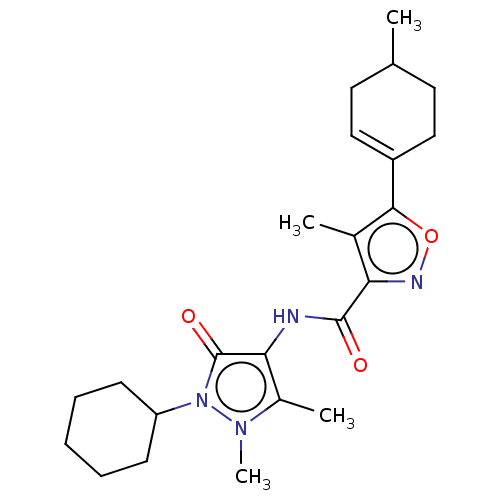
Affinity DataIC50: 0.900nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of ALK2 G328V mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of ALK2 G328V mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of ALK2 R258G mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2.5nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of ALK2 G328V mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.53nMAssay Description:Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of ALK2 R258G mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of ALK2 G328V mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.77nMAssay Description:Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of ALK2 G328V mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5.70nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 5.79nMAssay Description:Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 G328V mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of ALK2 R258G mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair




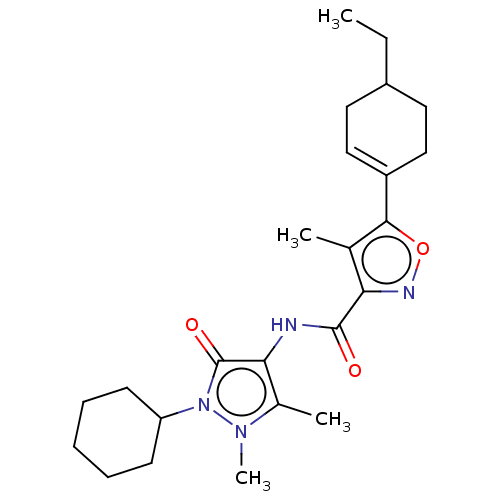
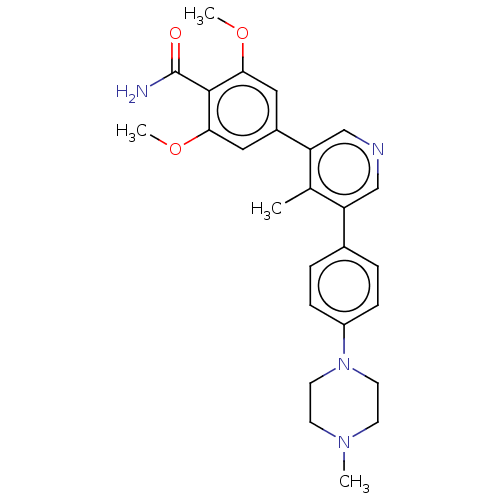
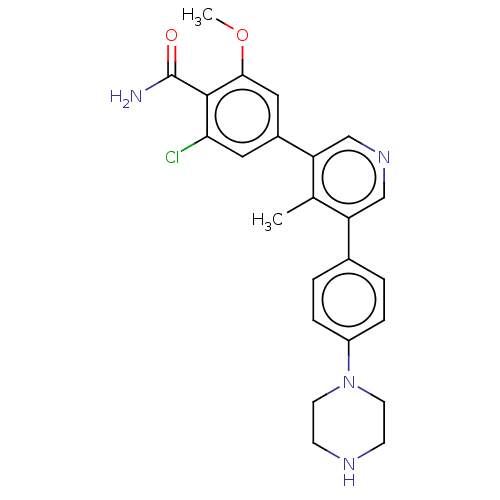


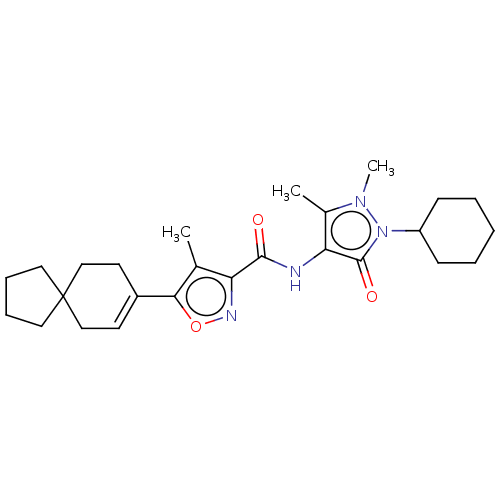


 3D Structure (crystal)
3D Structure (crystal)