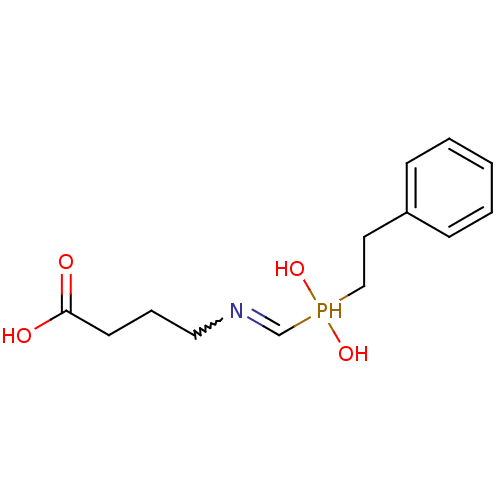
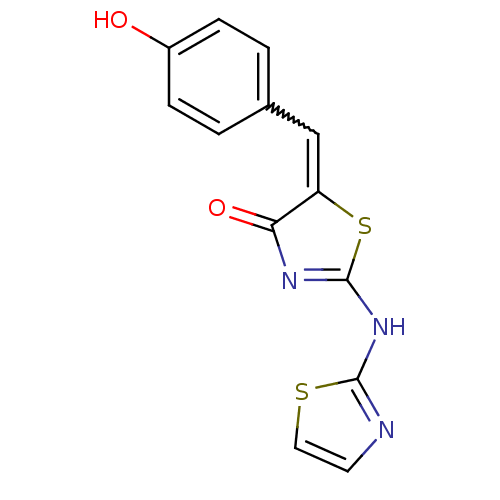
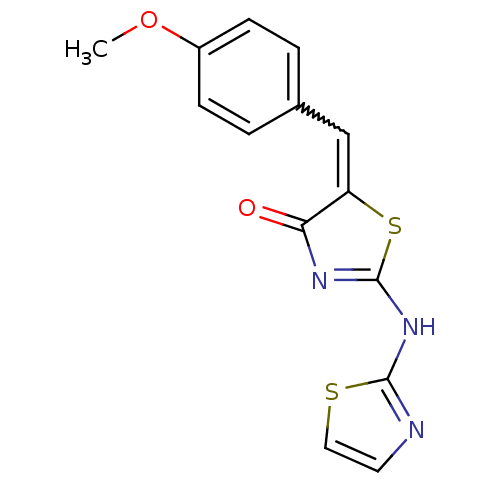
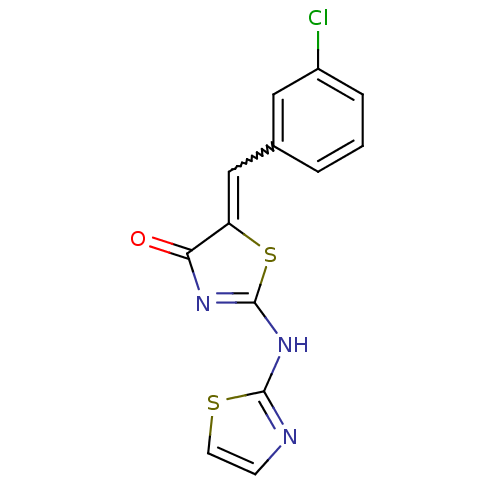
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
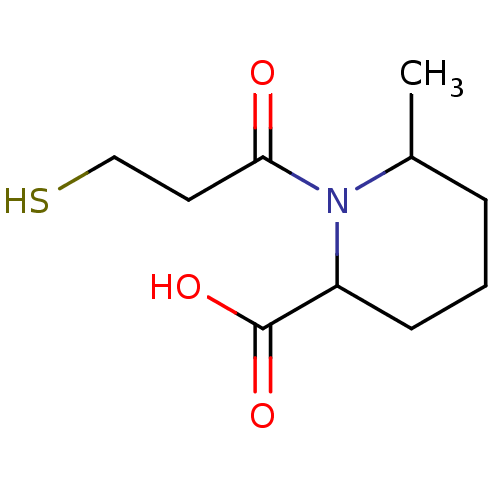
Affinity DataIC50: 0.100nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidneyMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
TargetNeprilysin(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
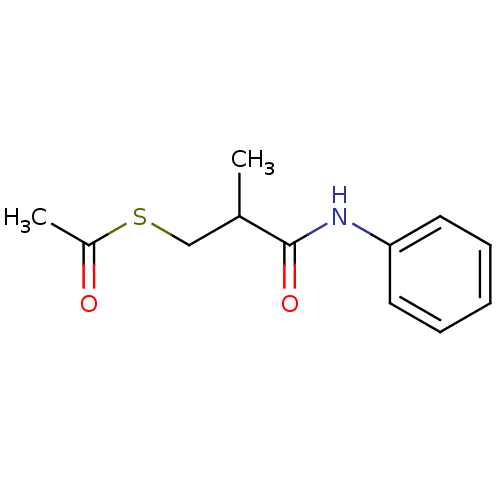
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from human blood serumMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetNeprilysin(Rattus norvegicus (Rat))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
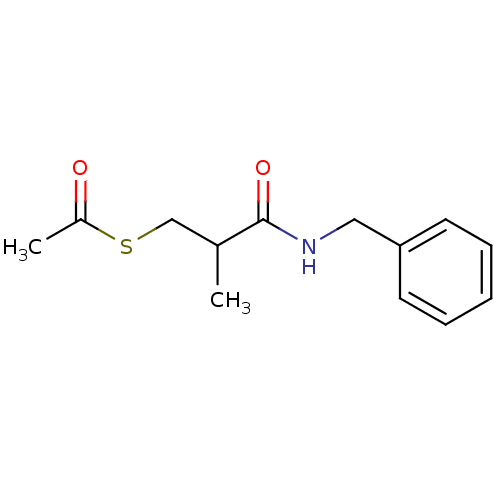
Affinity DataIC50: 200nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidneyMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
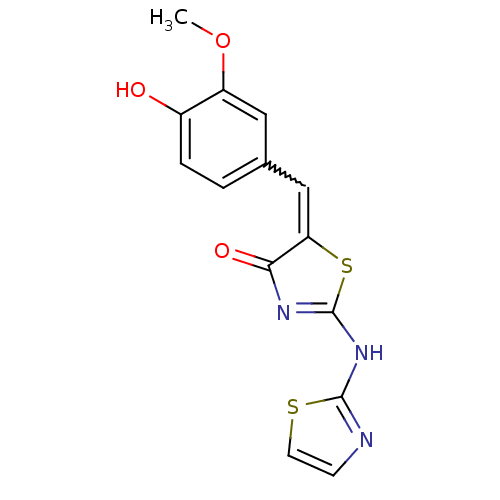
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
TargetNeprilysin(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from human blood serumMore data for this Ligand-Target Pair
TargetNeprilysin(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from human blood serumMore data for this Ligand-Target Pair
Affinity DataIC50: 8.91E+4nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.95E+4nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
Affinity DataIC50: 1.15E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.16E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.22E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+5nMpH: 8.0 T: 2°CAssay Description:The COX enzyme activities were measured using the COX Inhibitor Screening Assay kit provided by Cayman (Cayman, Chemical Co., Ann Arbor, MI). The ass...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+5nMpH: 8.0 T: 2°CAssay Description:The COX enzyme activities were measured using the COX Inhibitor Screening Assay kit provided by Cayman (Cayman, Chemical Co., Ann Arbor, MI). The ass...More data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+5nMpH: 8.0 T: 2°CAssay Description:The COX enzyme activities were measured using the COX Inhibitor Screening Assay kit provided by Cayman (Cayman, Chemical Co., Ann Arbor, MI). The ass...More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
TargetNeprilysin(Rattus norvegicus (Rat))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetNeprilysin(Rattus norvegicus (Rat))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+5nMpH: 7.4 T: 2°CAssay Description:The tested compounds were added to the reaction mixture with soybean lipoxygenase. Enzyme reaction was initiated by the addition of sodium linoleate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.62E+5nMpH: 8.0 T: 2°CAssay Description:The COX enzyme activities were measured using the COX Inhibitor Screening Assay kit provided by Cayman (Cayman, Chemical Co., Ann Arbor, MI). The ass...More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidneyMore data for this Ligand-Target Pair
TargetNeprilysin(Rattus norvegicus (Rat))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membraneMore data for this Ligand-Target Pair
TargetNeprilysin(Rattus norvegicus (Rat))
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membraneMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)