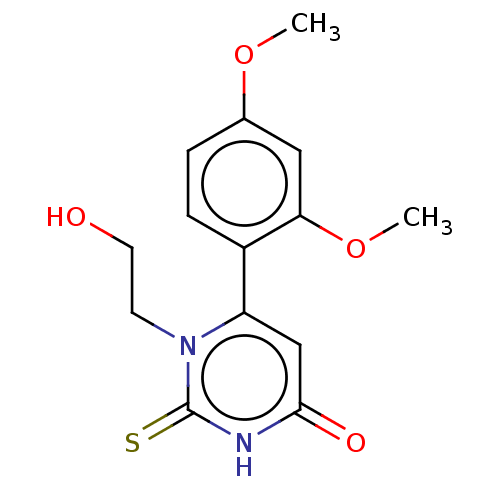
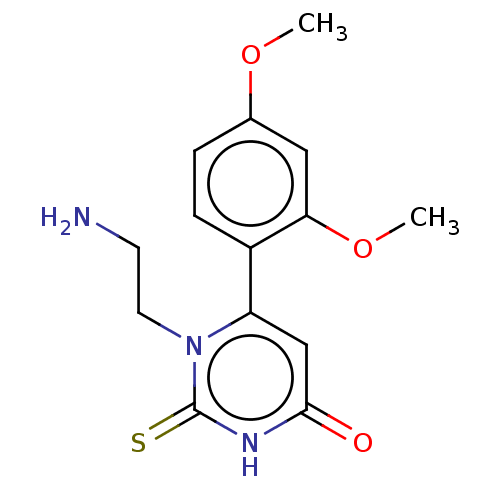
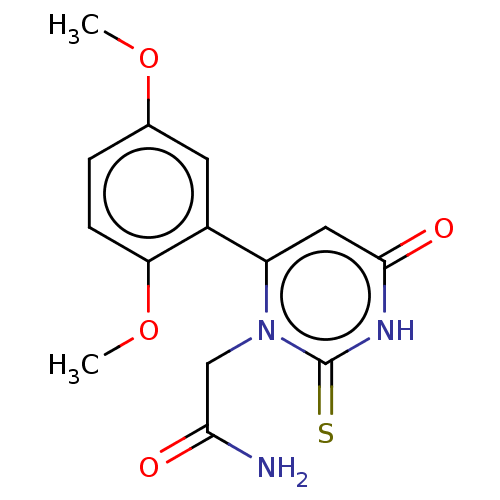
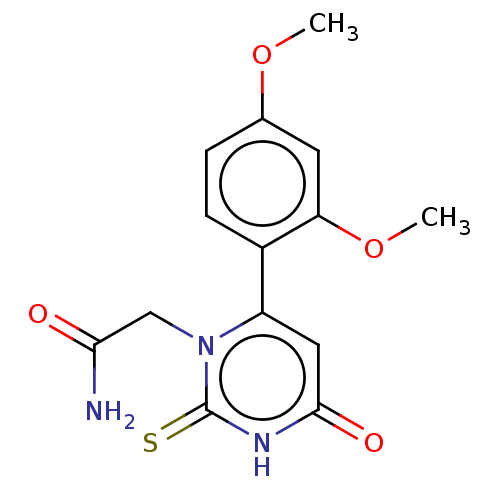
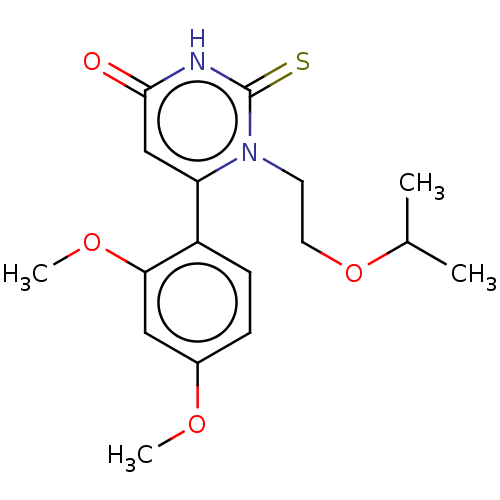
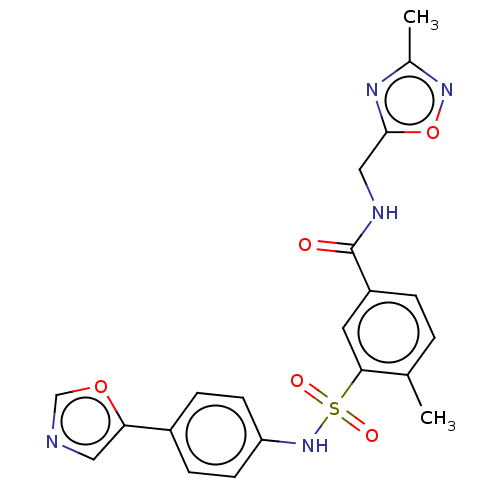
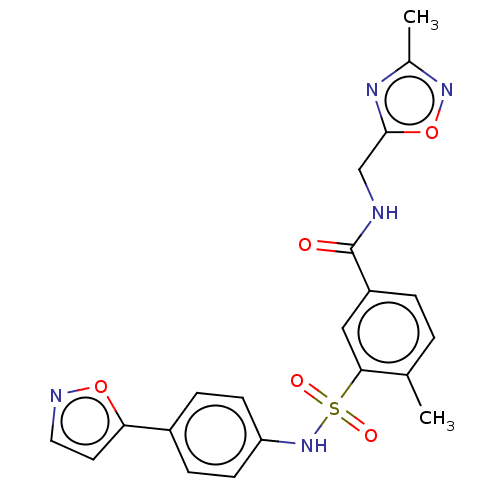
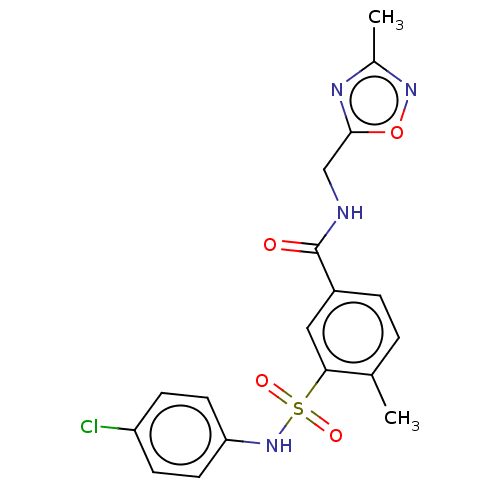
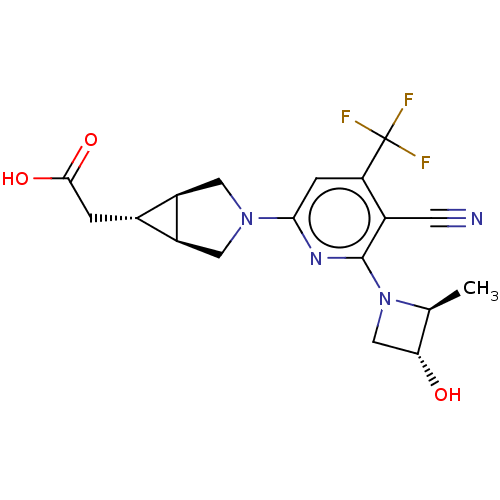
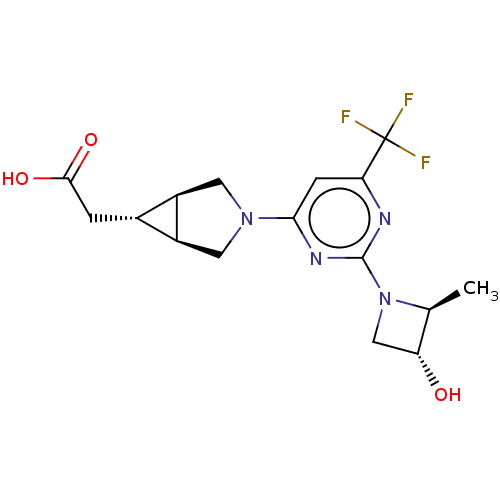
Affinity DataKi: 4.5nMAssay Description:Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ...More data for this Ligand-Target Pair
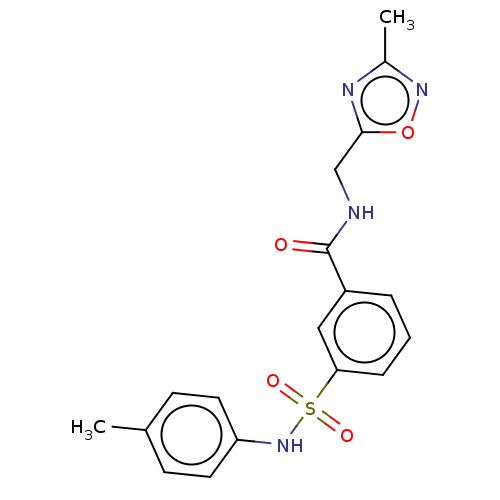
Affinity DataKi: 151nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
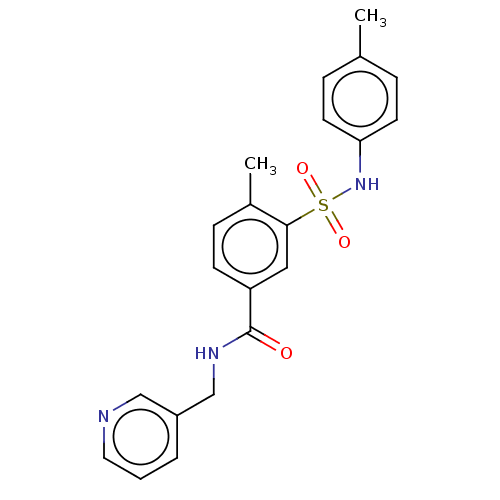
Affinity DataKi: 174nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
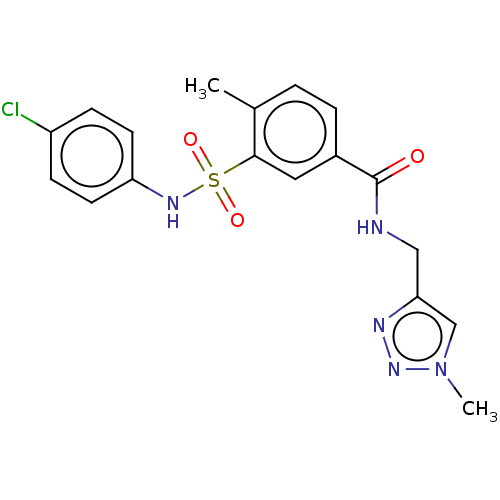
Affinity DataKi: 316nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
Affinity DataKi: 347nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
Affinity DataKi: 372nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
Affinity DataKi: 501nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenatesMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assayMore data for this Ligand-Target Pair