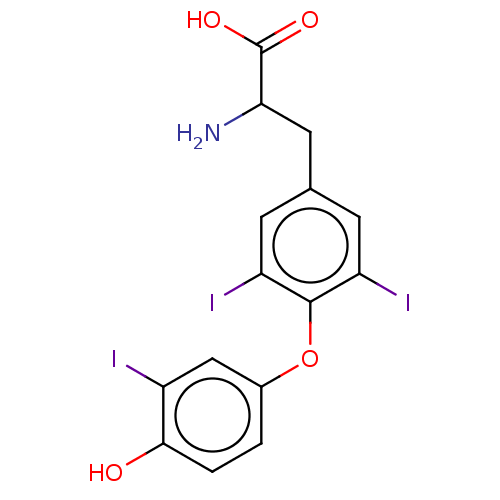
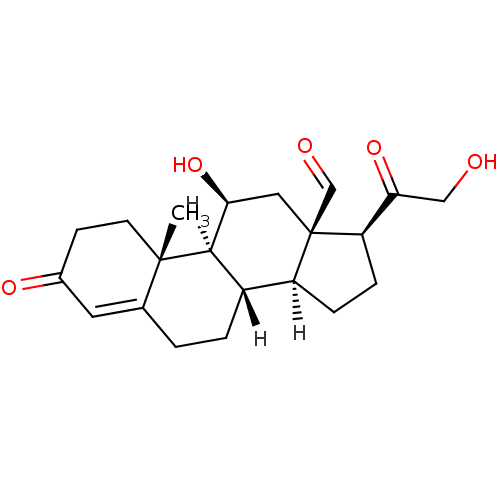
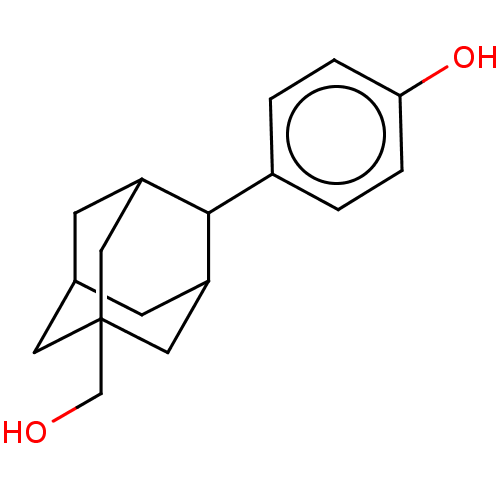
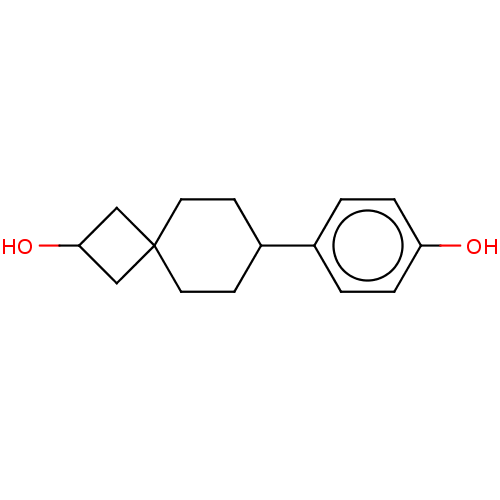
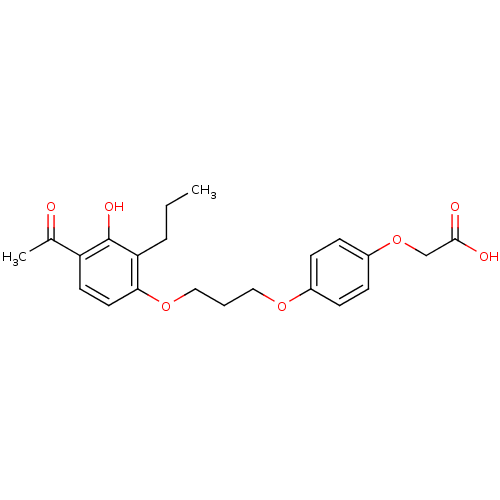
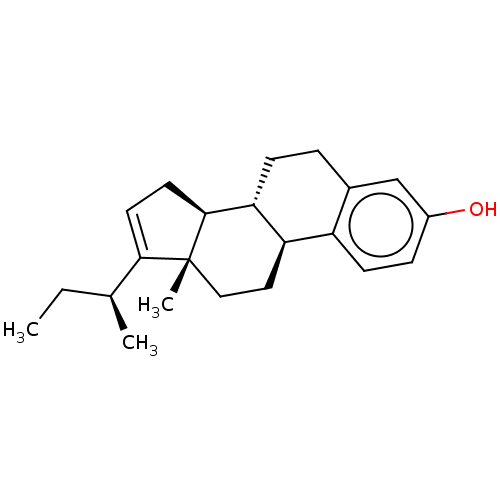
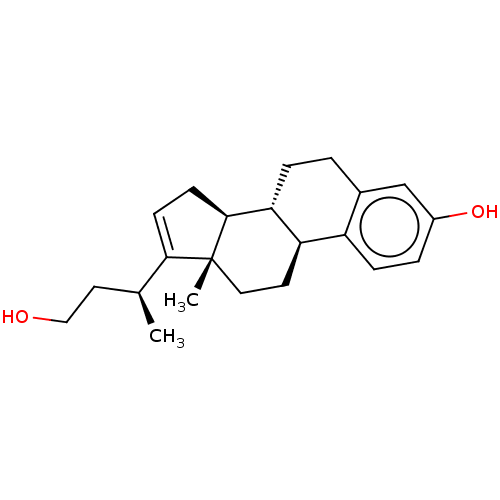
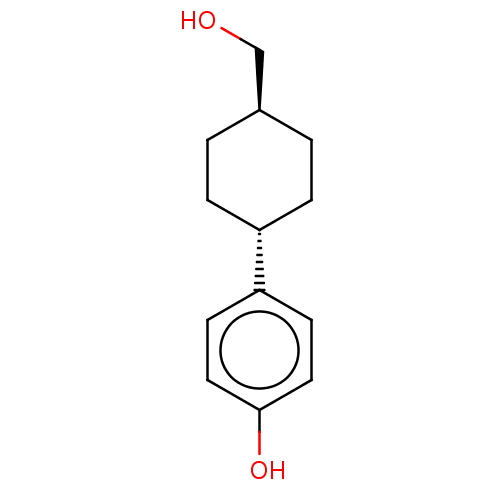
TargetDual specificity protein phosphatase 5 [180-384](Homo sapiens (Human))
Texas Wesleyan University
Texas Wesleyan University
Affinity DataKi: 25nM ΔG°: -43.4kJ/mole IC50: 4.40E+4nMT: 2°CAssay Description:For the 96-well plate validation assay, sodium orthovanadate (Sigma Aldrich) was utilized as a positive control for inhibition [Swarup et al., Bioche...More data for this Ligand-Target Pair
Affinity DataKi: 26nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 42nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 100nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 202nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 620nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 7.90E+3nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataIC50: 0.0460nMAssay Description:Antagonist activity at ERbeta (unknown origin) by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0953nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused VDR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lac...More data for this Ligand-Target Pair
Affinity DataIC50: 0.103nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused TRbeta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed...More data for this Ligand-Target Pair
Affinity DataIC50: 0.107nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused ERalpha (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.236nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused PR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact...More data for this Ligand-Target Pair
Affinity DataIC50: 0.302nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused androgen receptor (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.305nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused MR (unknown origin) ligand binding domain expressed in UAS-bla H cells assessed as beta-lactamase t...More data for this Ligand-Target Pair
Affinity DataIC50: 0.579nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused ERbeta (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Agonist activity at ERalpha (unknown origin) by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused GR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Marquette University
Curated by ChEMBL
Marquette University
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Agonist activity at GAL4 DNA-binding domain fused PPARdelta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells asses...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte...More data for this Ligand-Target Pair
Affinity DataIC50: 88nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 108nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 111nMAssay Description:Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte...More data for this Ligand-Target Pair
Affinity DataIC50: 145nMAssay Description:Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 191nMAssay Description:Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 275nMAssay Description:Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte...More data for this Ligand-Target Pair
Affinity DataIC50: 484nMAssay Description:Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Concordia University of Wisconsin
Concordia University of Wisconsin
Affinity DataIC50: 1.10E+3nMpH: 7.0 T: 2°CAssay Description:Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:This assay was done with full-length protein (containing both domains), andusing pERK as substrate.More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Concordia University of Wisconsin
Concordia University of Wisconsin
Affinity DataIC50: 2.10E+3nMpH: 7.0 T: 2°CAssay Description:Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)