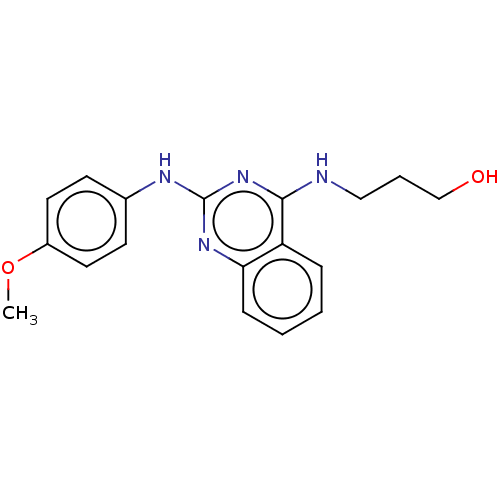
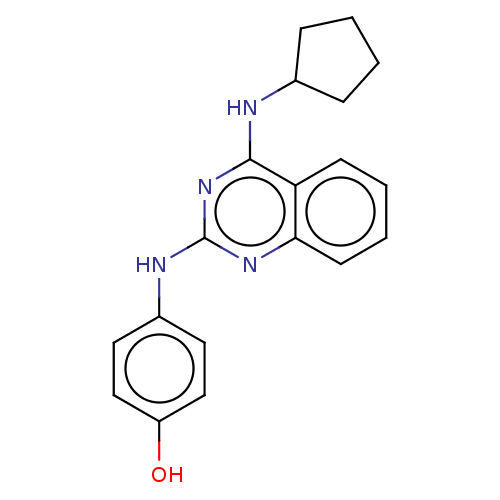
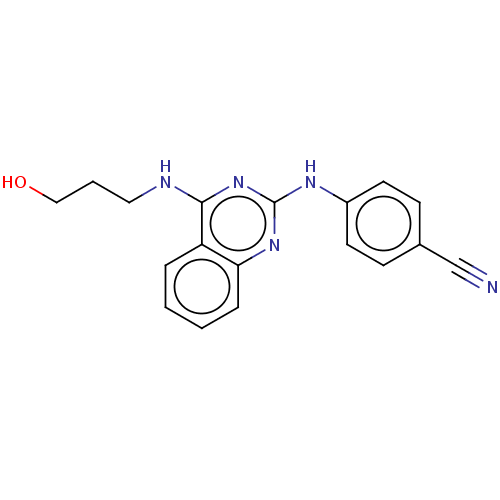
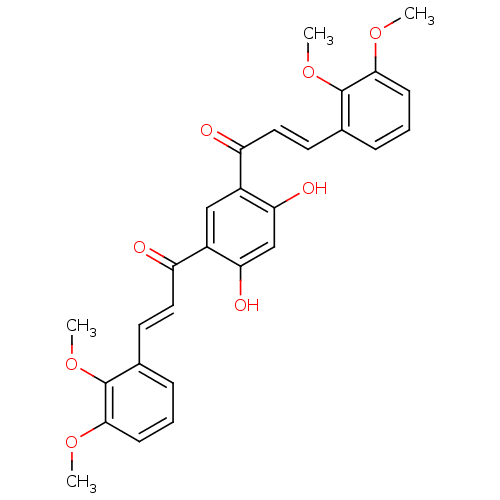
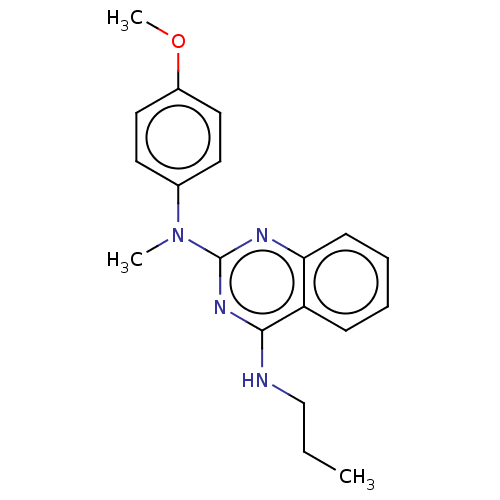
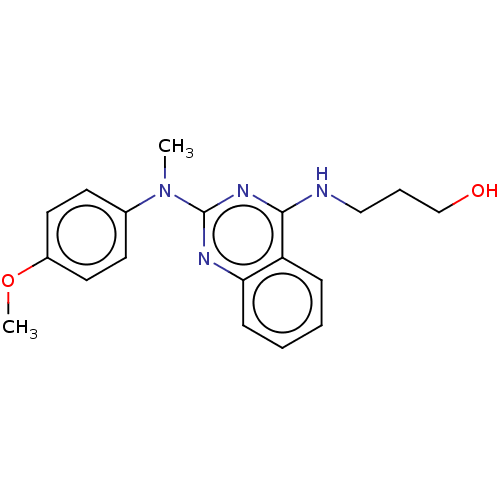
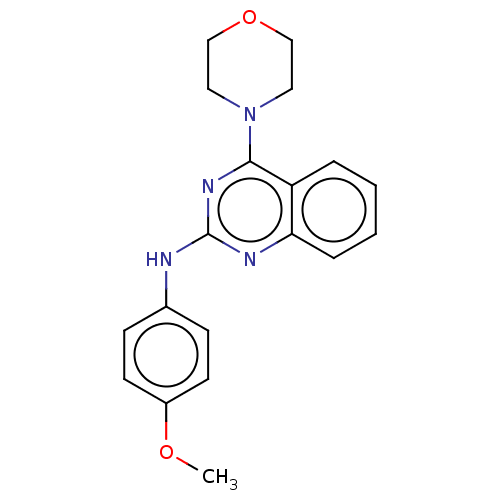
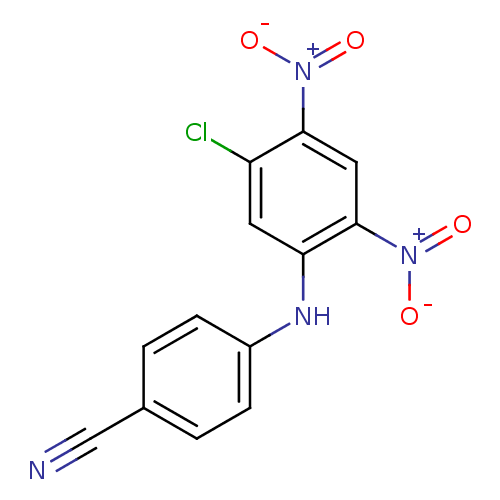
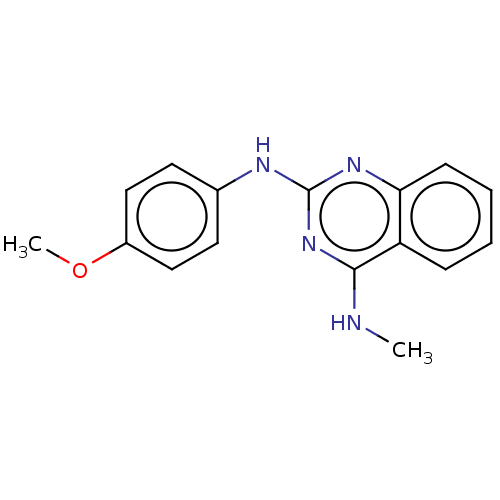
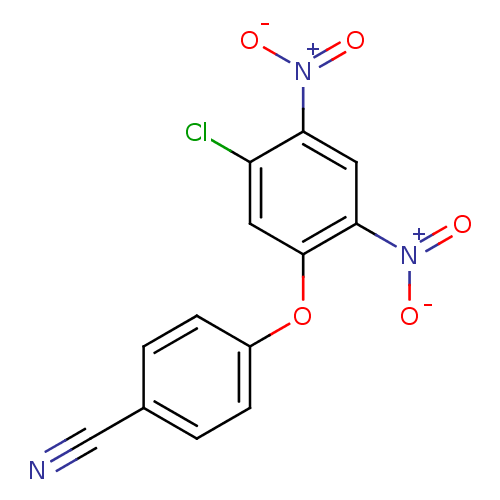
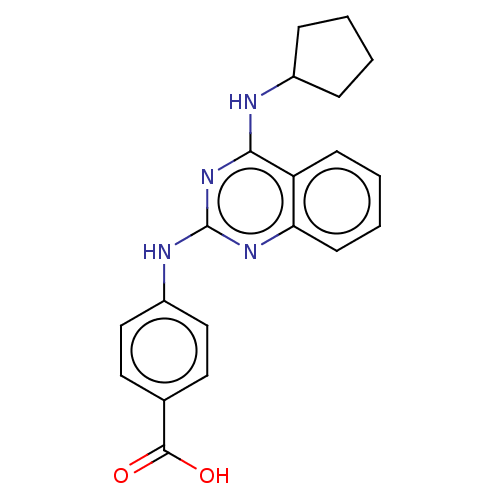
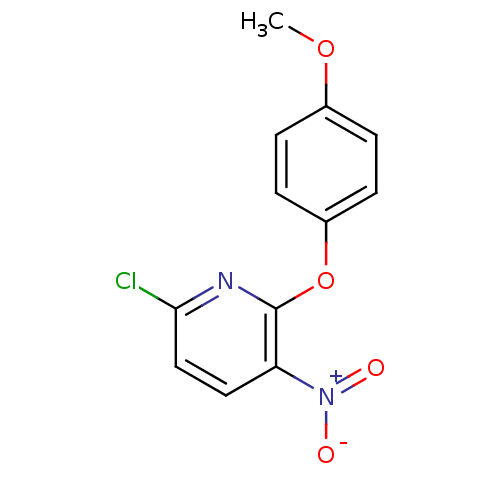
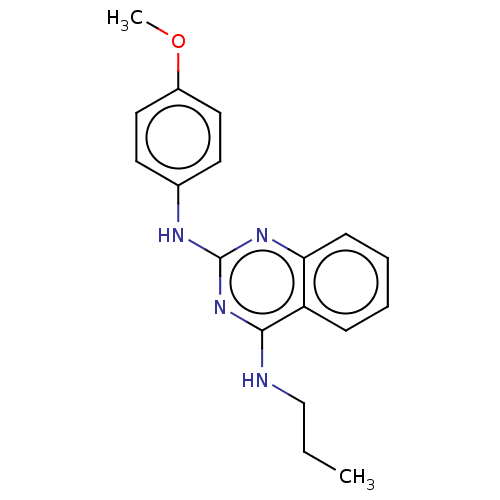
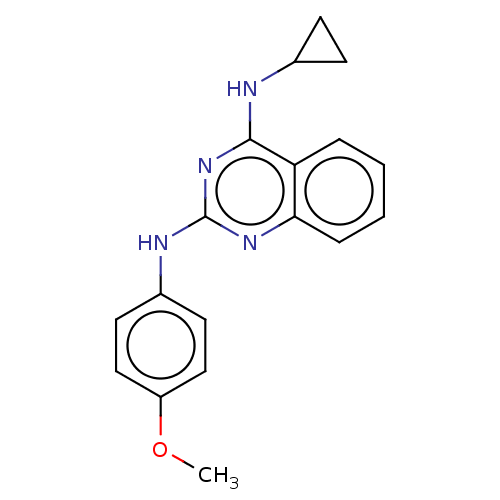
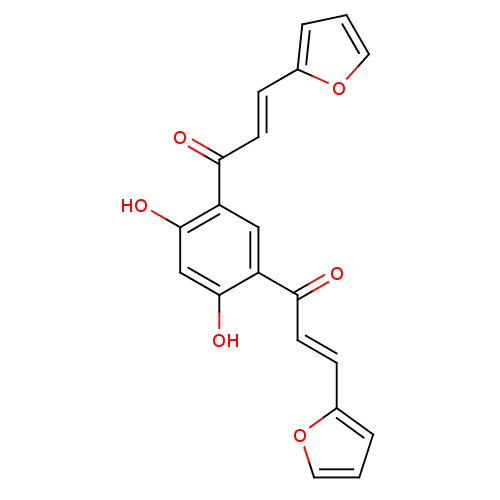
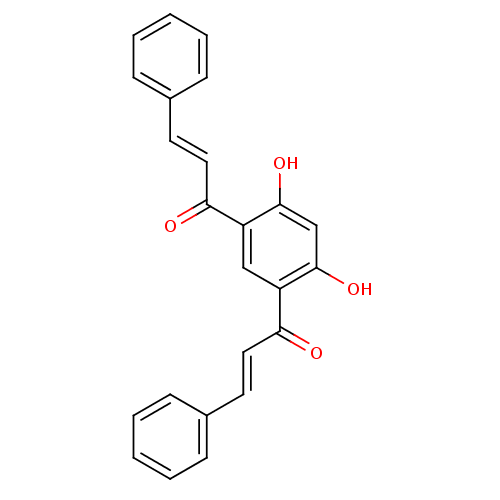
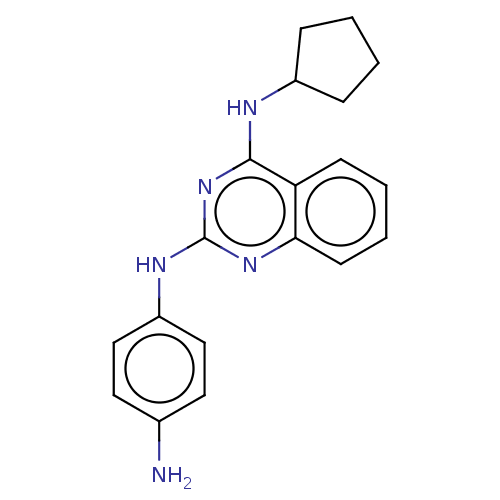
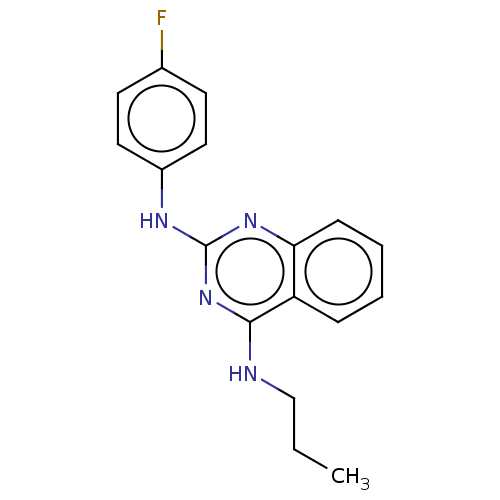
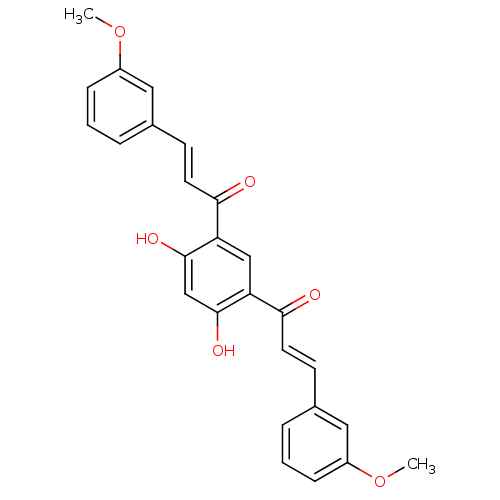
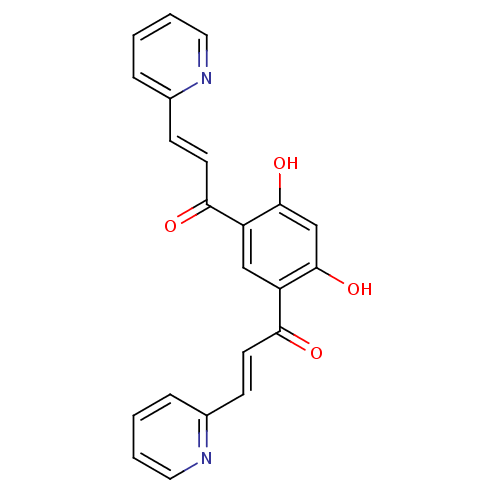
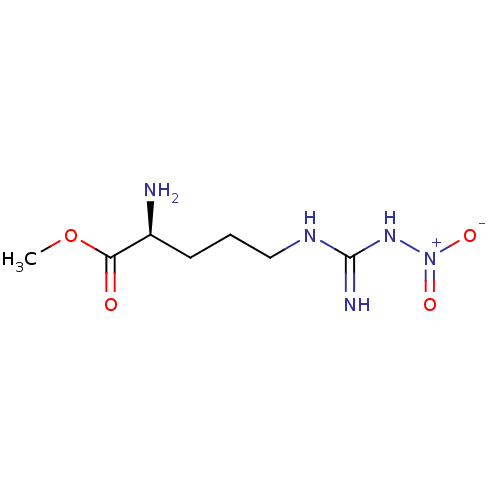
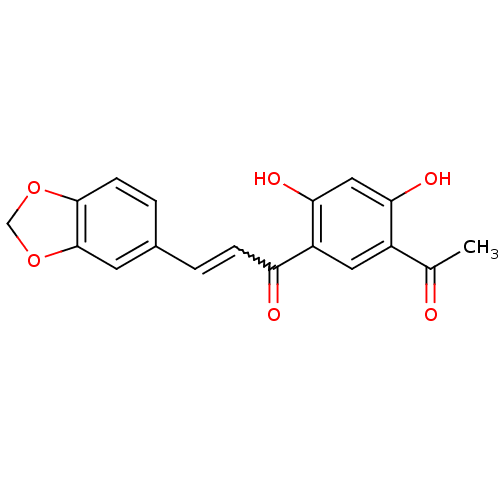
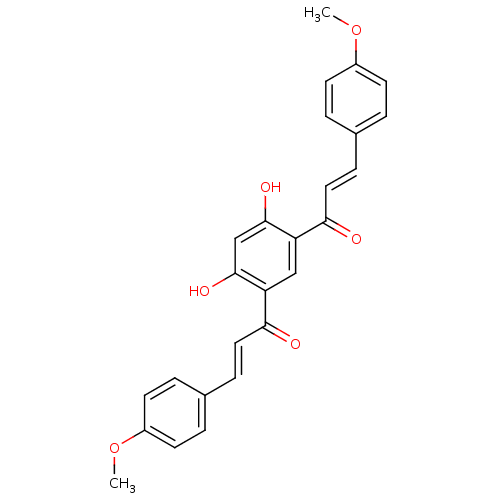
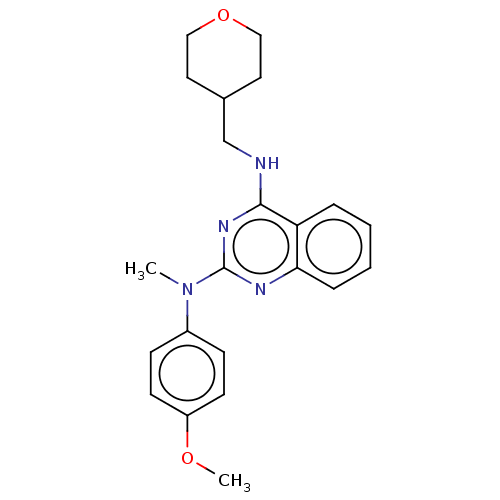
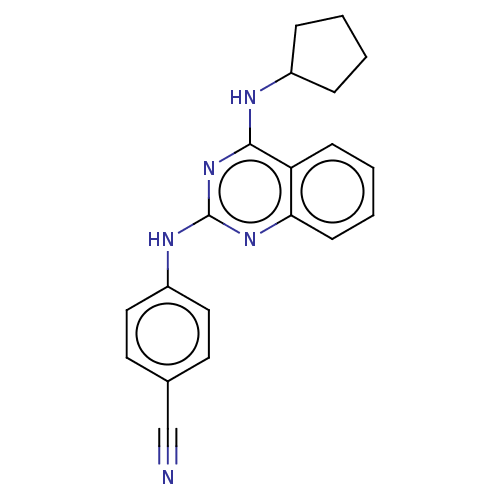
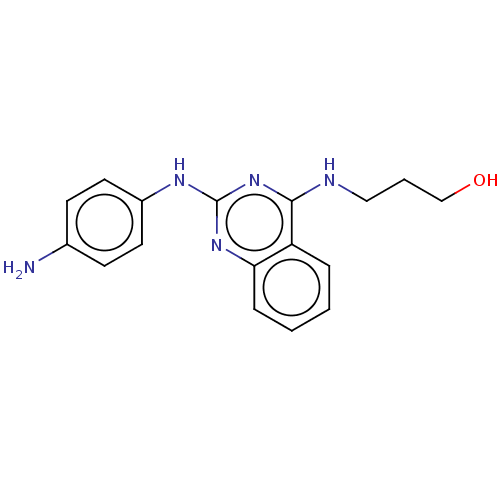
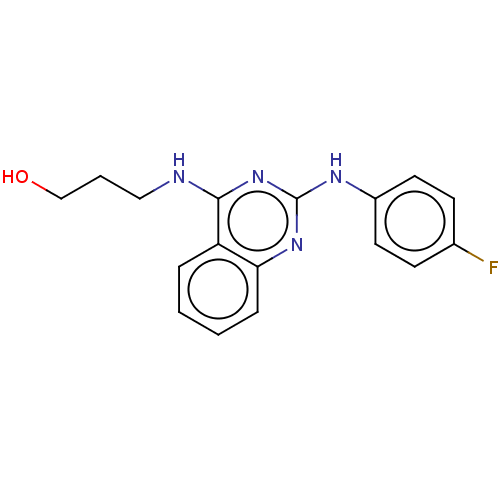
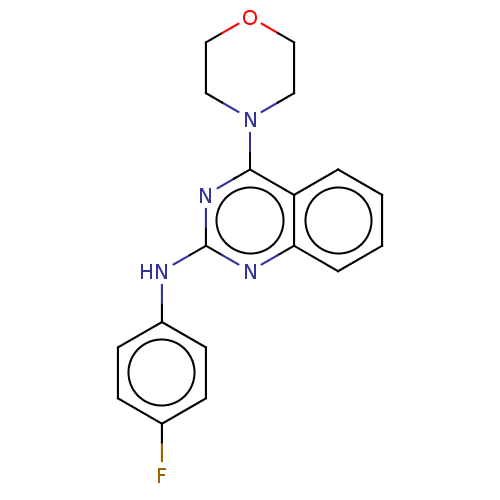
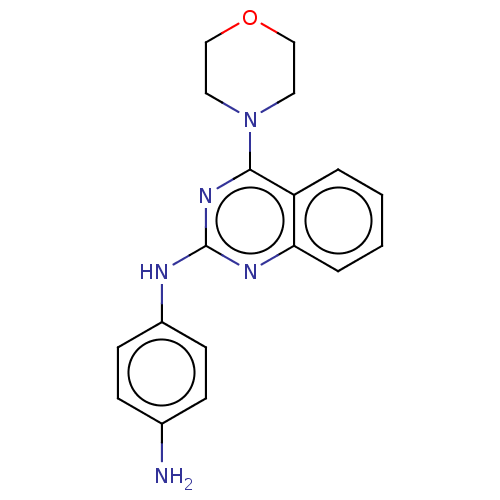
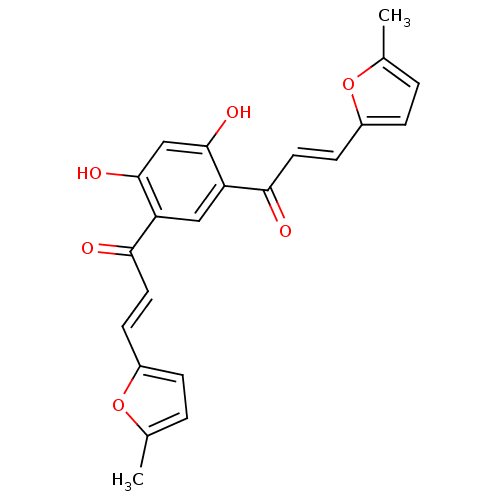
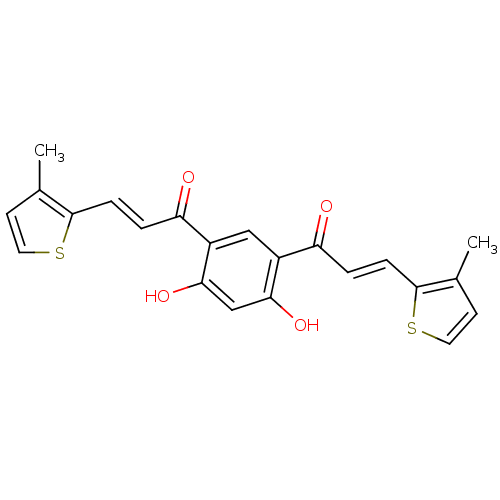
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
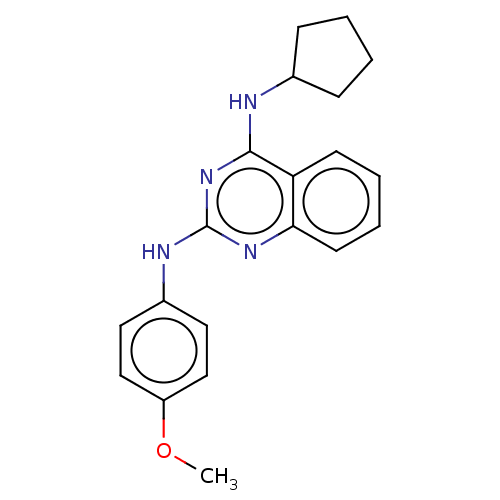
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 680nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
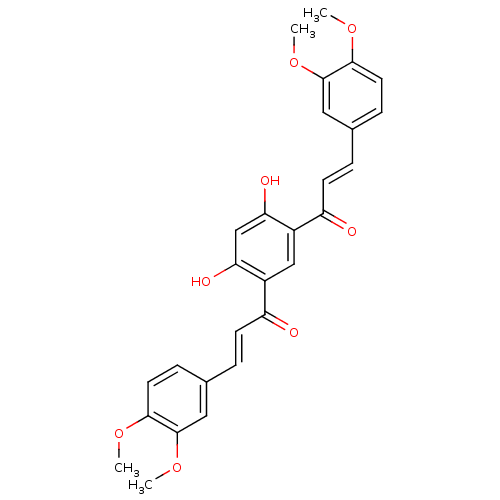
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
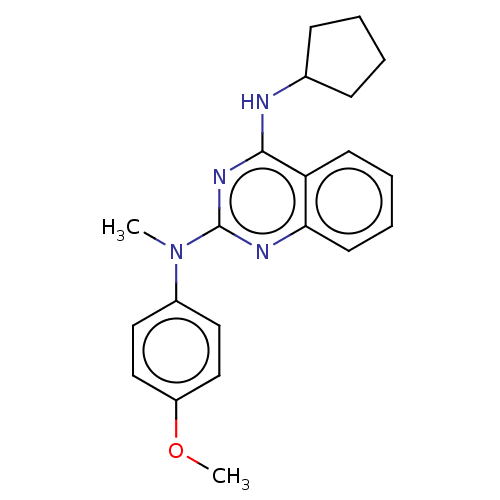
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.08E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.14E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.24E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.72E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 1.82E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.84E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.01E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.06E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.25E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.43E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.56E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 4.17E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.44E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 7.04E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 7.27E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 7.84E+3nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 8.09E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 8.65E+3nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of N-terminal His-tagged Mer kinase (588 to 855 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells measured every mi...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.33E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.57E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of MERTK (unknown origin) using EFPIYDFLPAKKK-CONH2 as substrate and ATP after 180 mins by microfluidic capillary electrophoresis methodMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Beijing Institute of Pharmacology& Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 3.63E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 4.33E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair