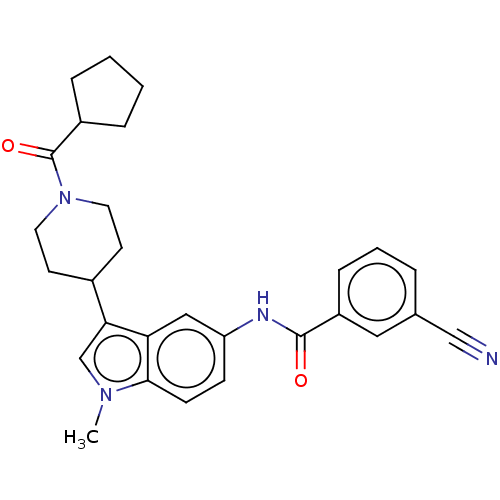
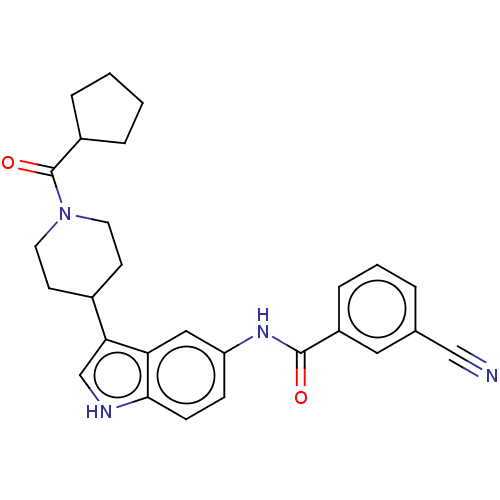
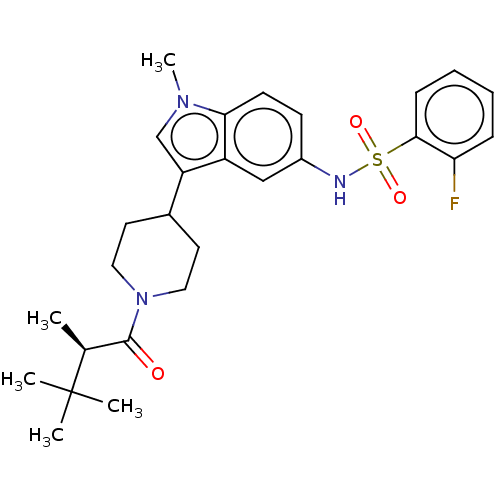
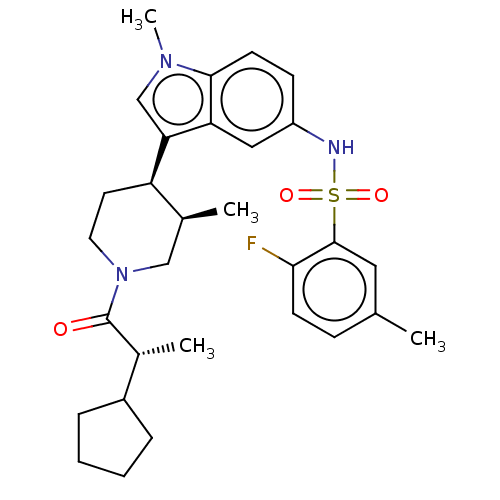
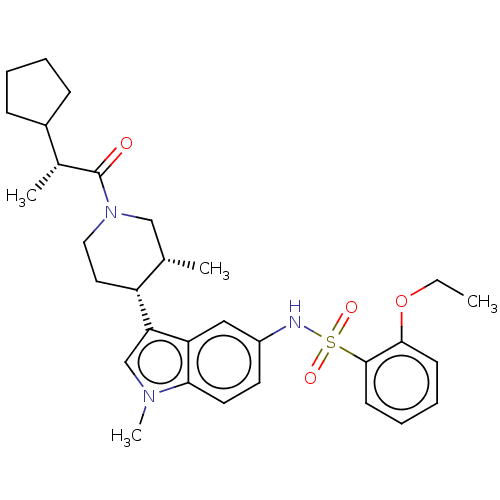
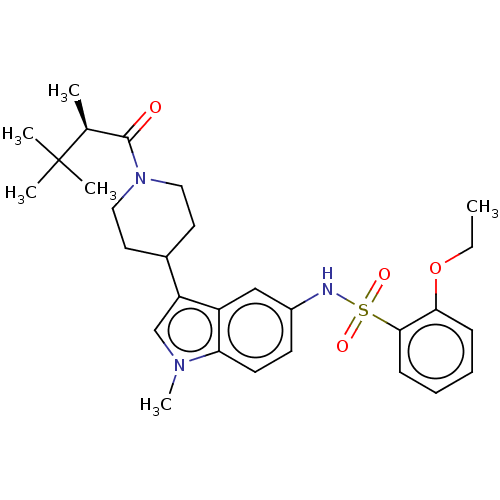
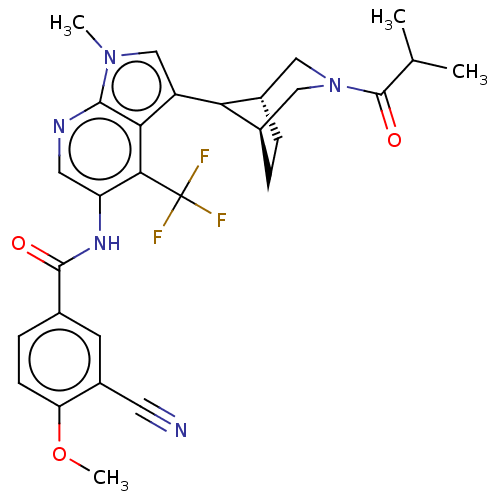
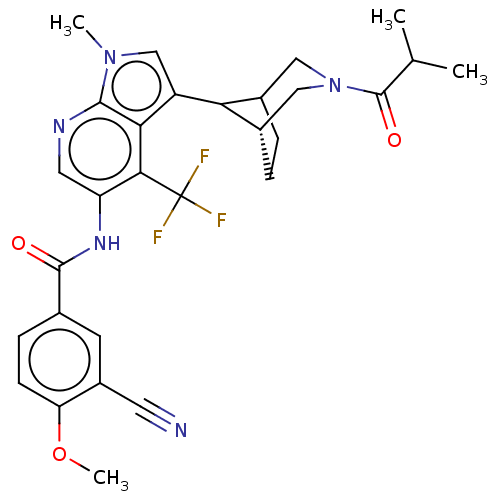
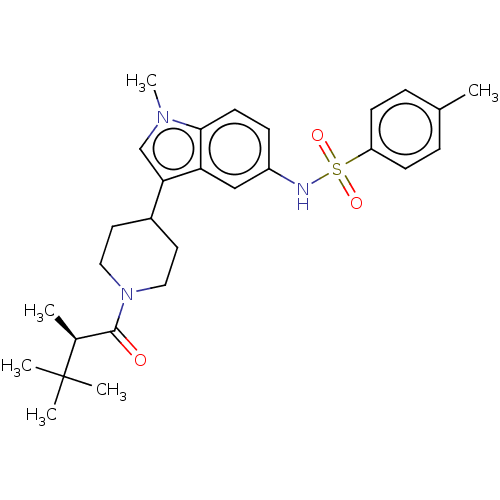
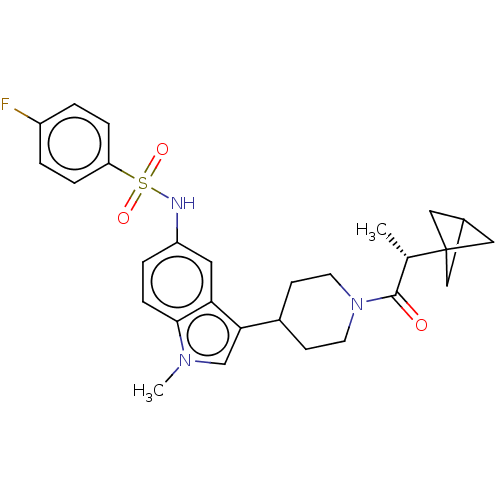
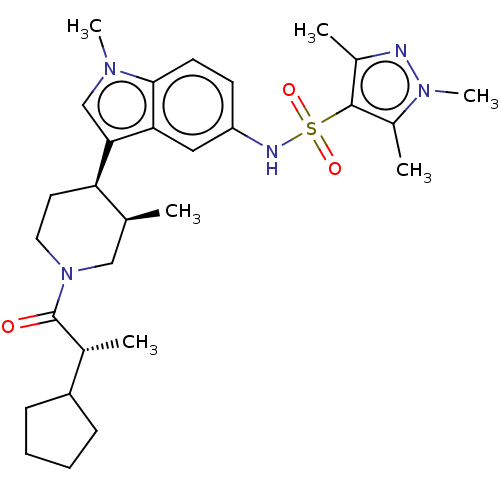
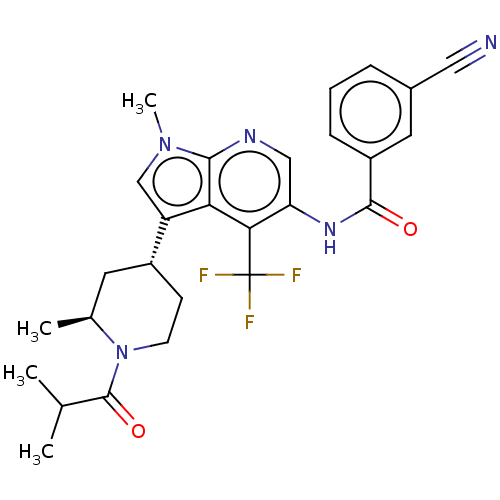
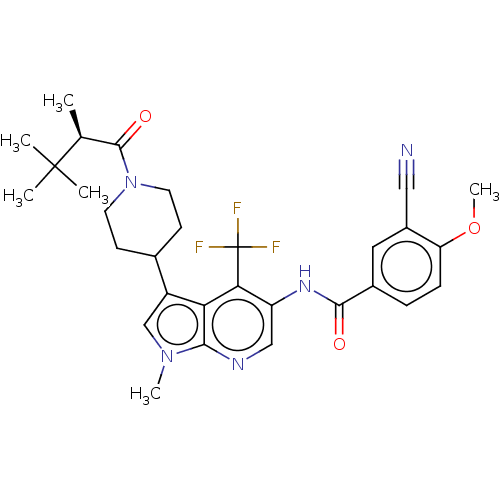
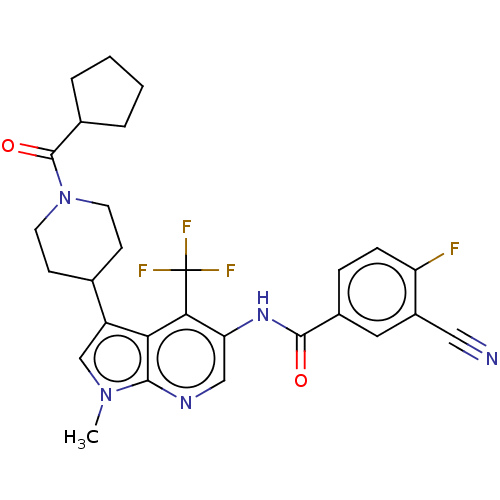
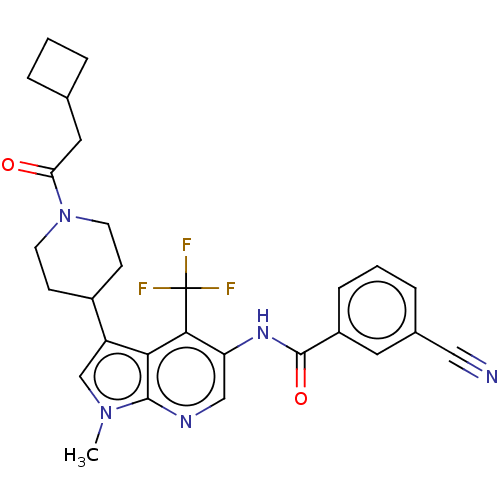
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
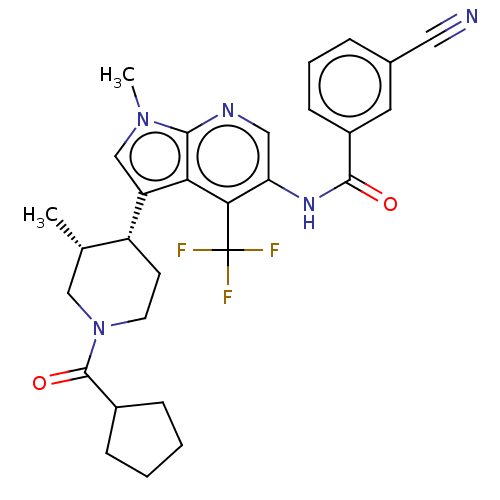
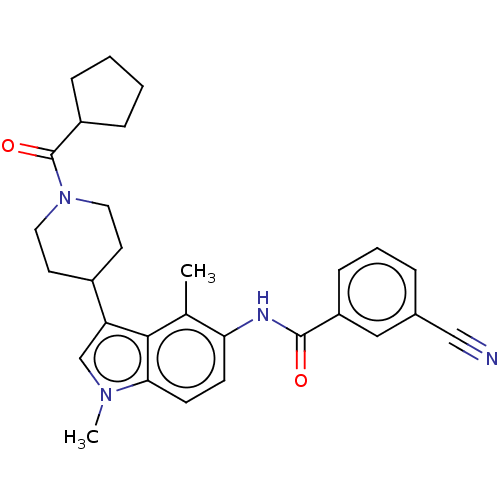
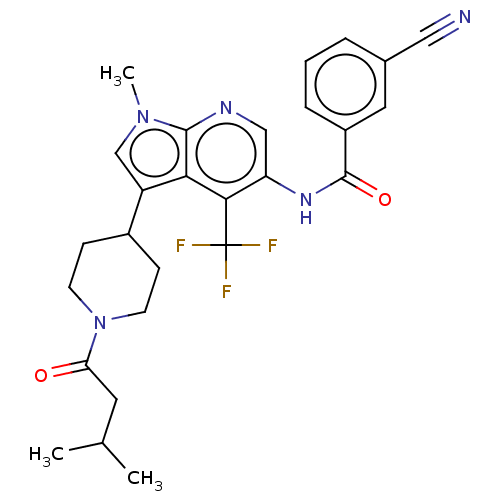
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
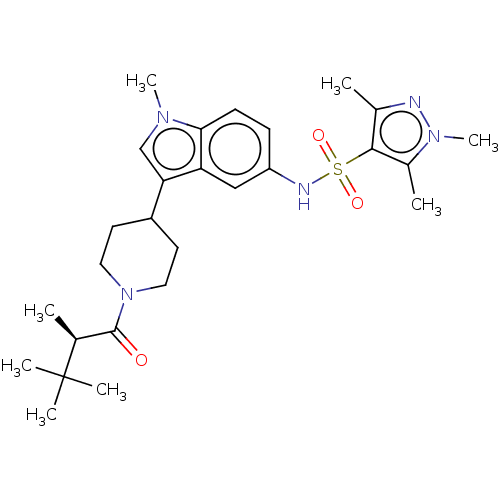
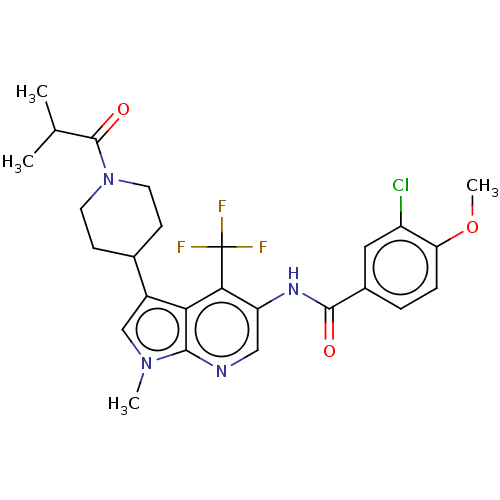
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
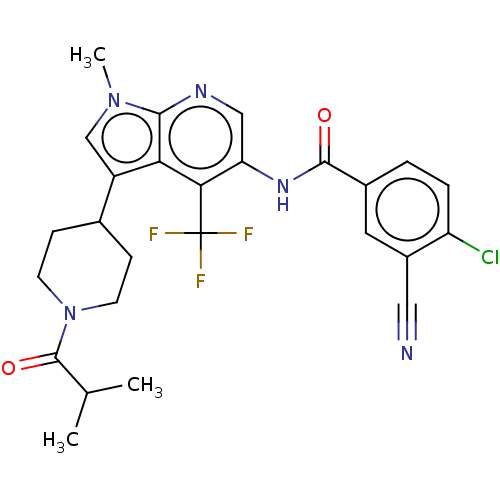
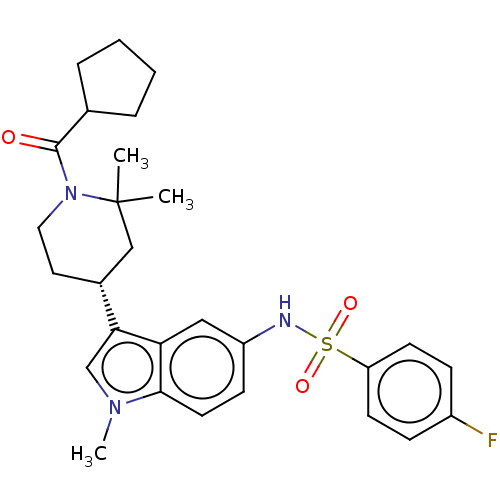
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 0.900nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.20nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.5nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.10nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.70nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.80nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.20nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISAMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.5nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.80nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
Affinity DataIC50: 3.90nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair

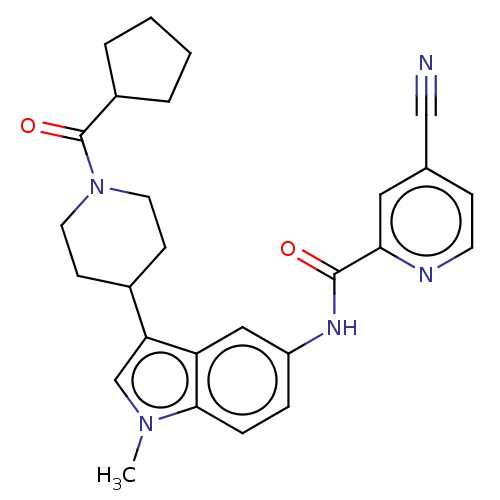
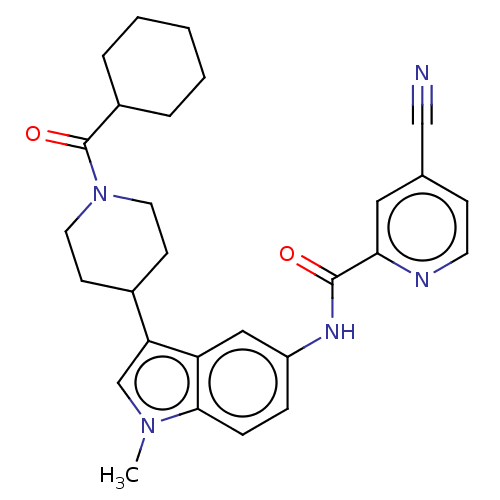
 3D Structure (crystal)
3D Structure (crystal)