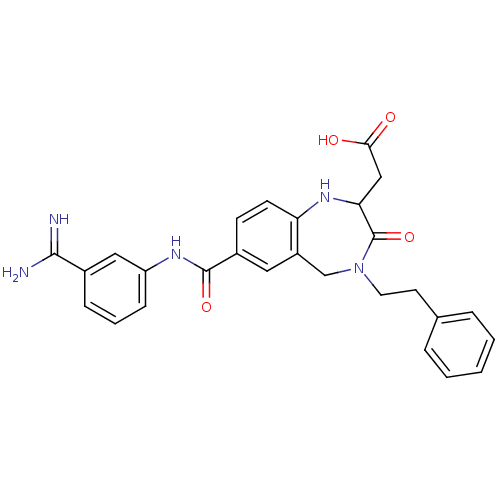
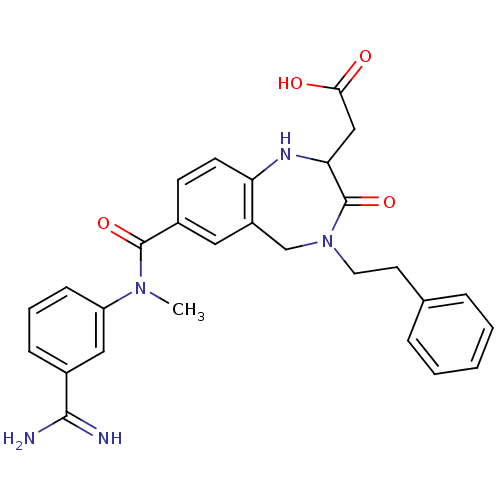
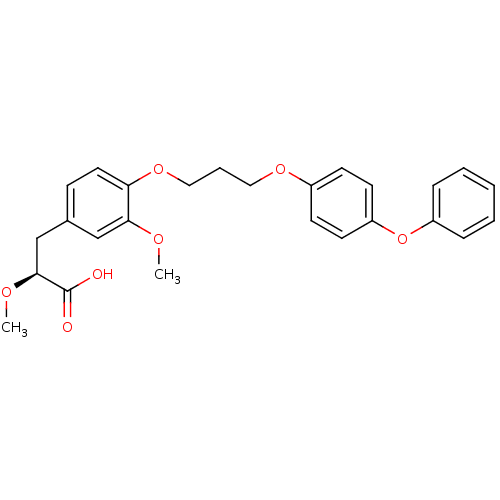
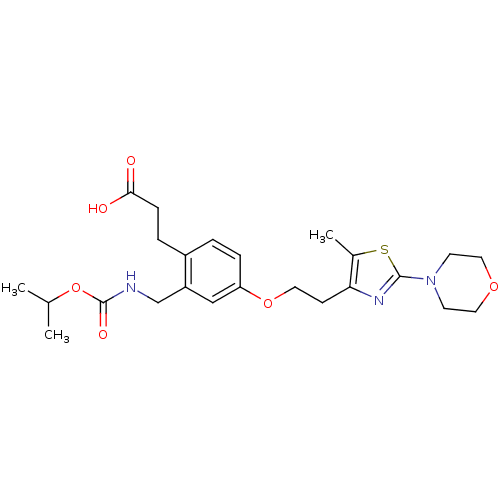
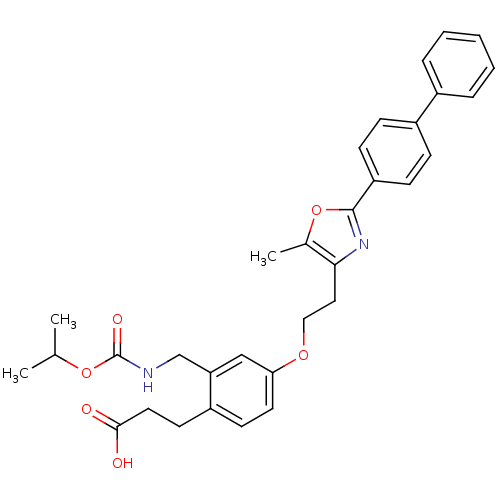
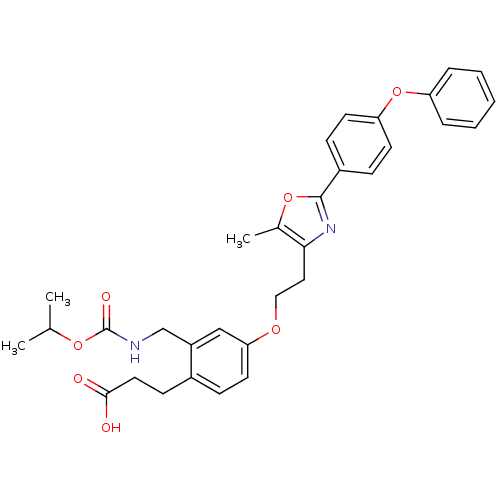
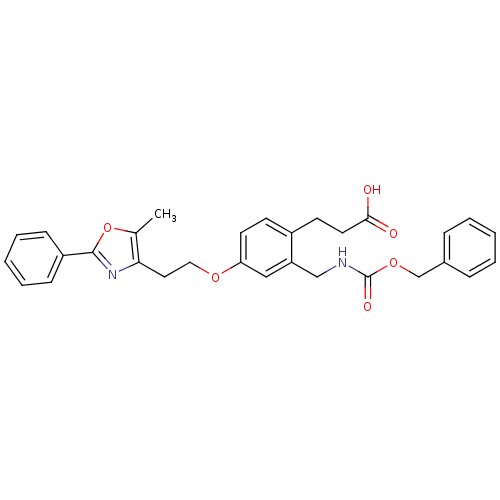
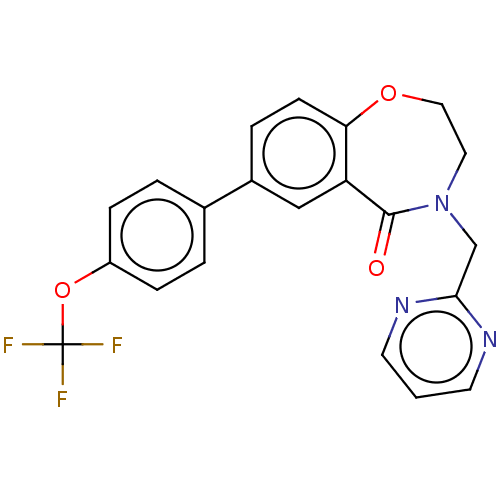
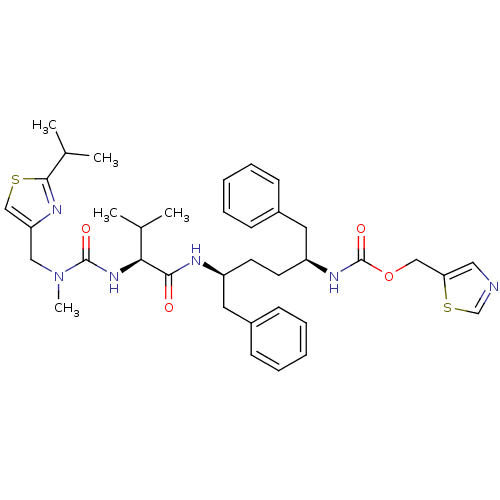
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
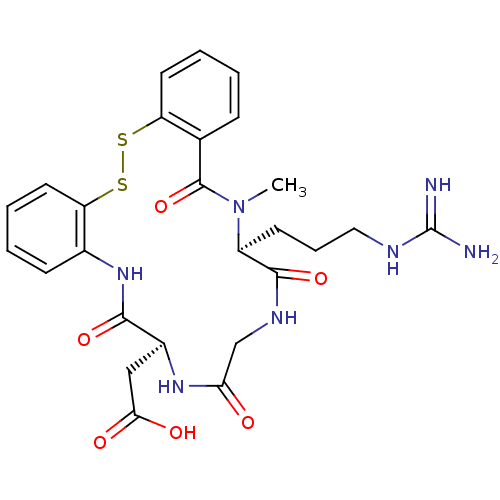
Affinity DataKi: 1.5nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-IIb/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.53E+3nMAssay Description:Inhibition of binding to purified integrin alphaIIb-beta3 of human plateletsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
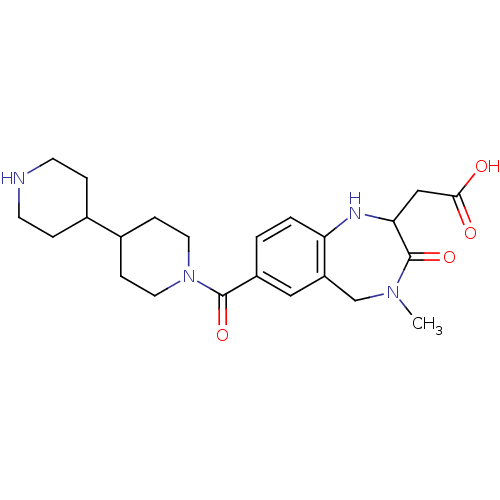
Affinity DataIC50: 5nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator-activated receptor gammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 91nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at -80 mV resting membrane potential by electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at 3 Hz frequency by manual patch clamp techniqueMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 146nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 161nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at 1 Hz frequency by manual patch clamp techniqueMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 309nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 441nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 449nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 516nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 568nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at -120 mV resting membrane potential by electroph...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at 0.1 Hz frequency by manual patch clamp techniqu...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 706nMAssay Description:Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Co
Curated by ChEMBL
Eli Lilly and Co
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Mean inhibitory concentration against human peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair

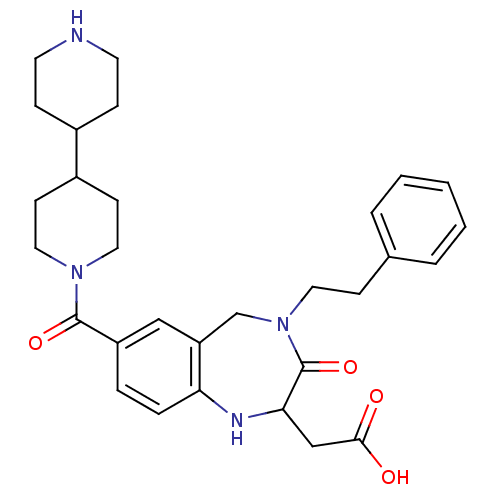

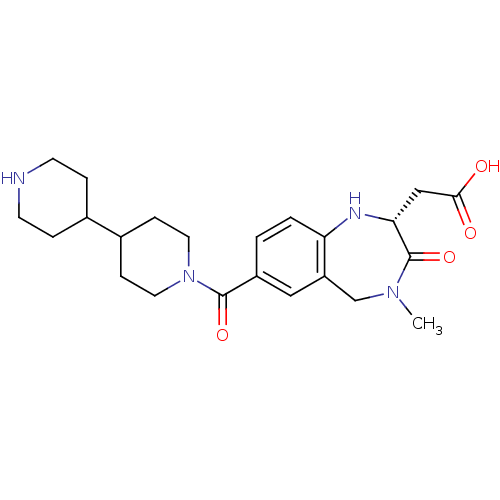
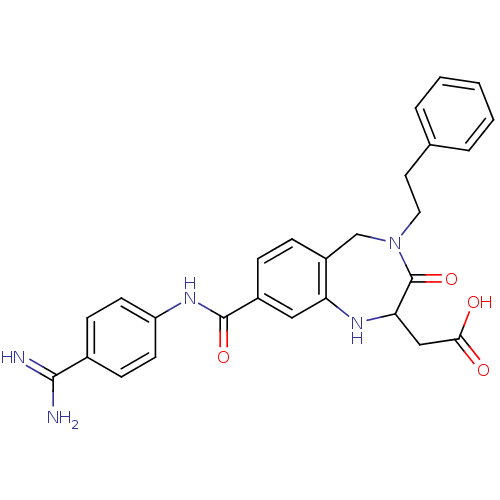
 3D Structure (crystal)
3D Structure (crystal)