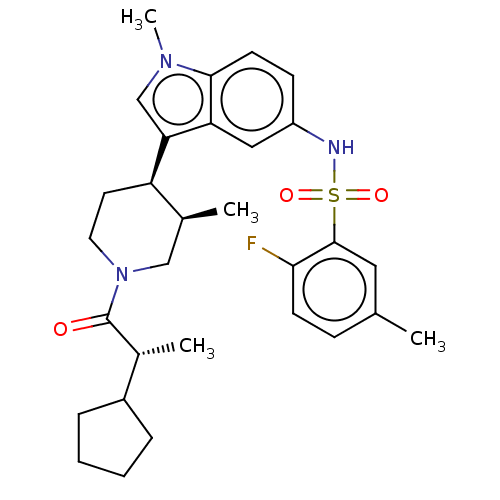
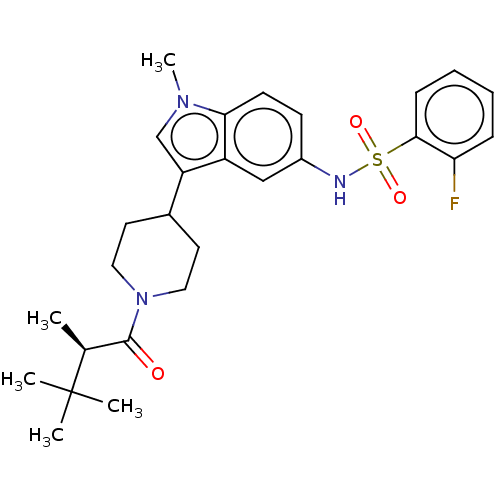
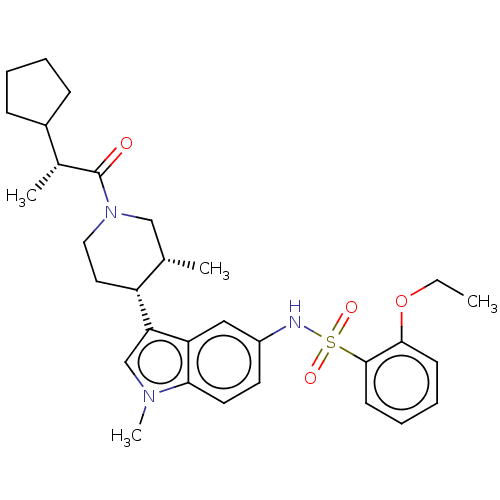
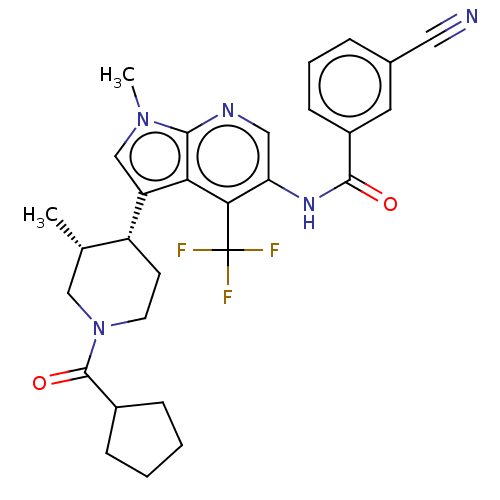
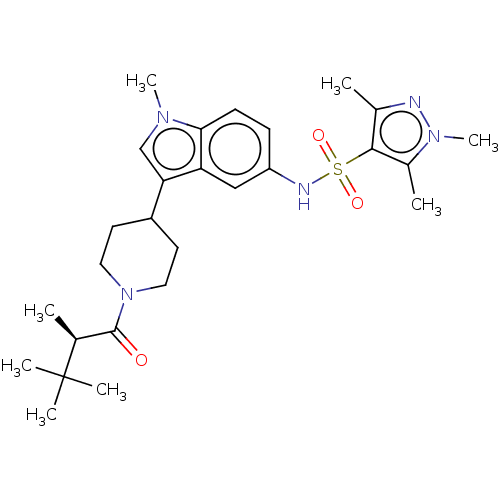
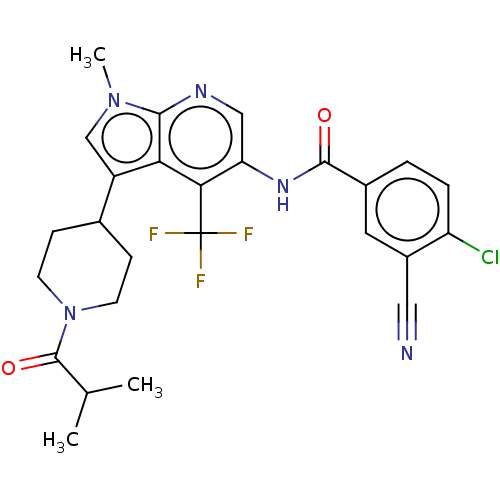
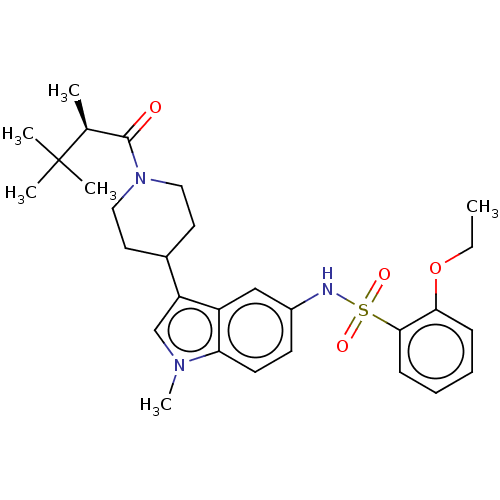
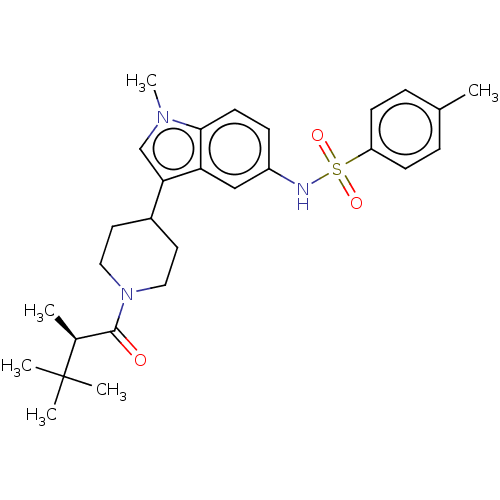
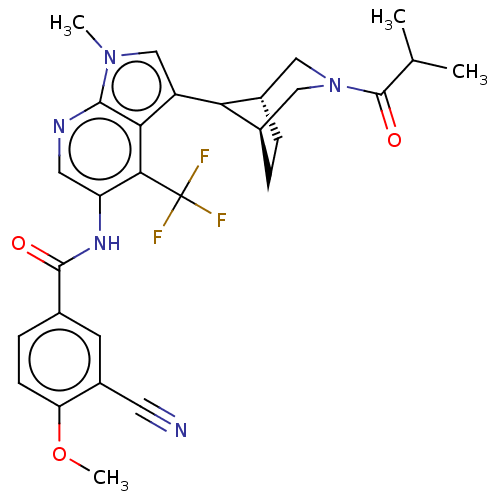
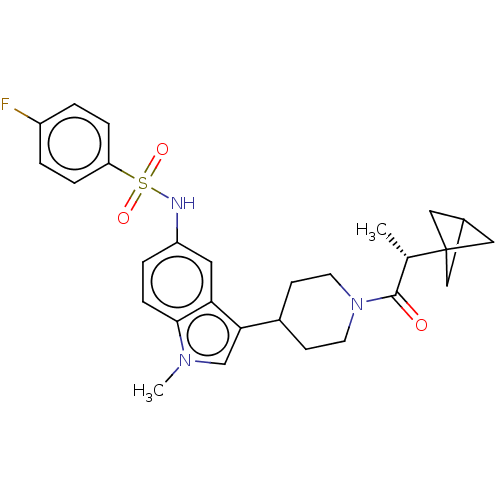
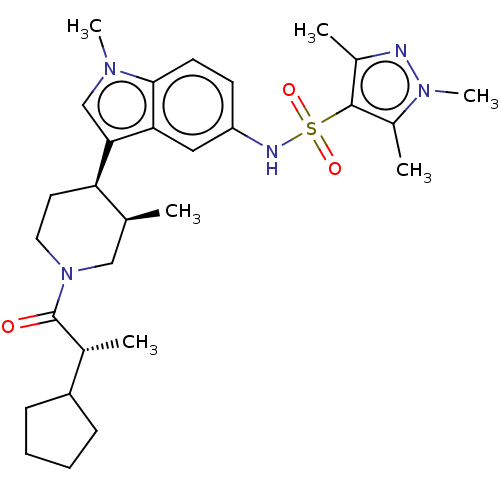
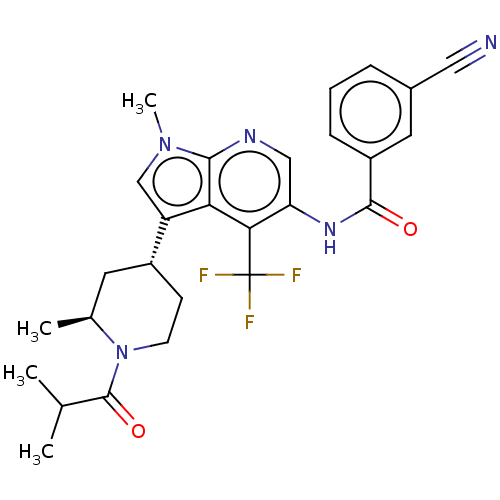
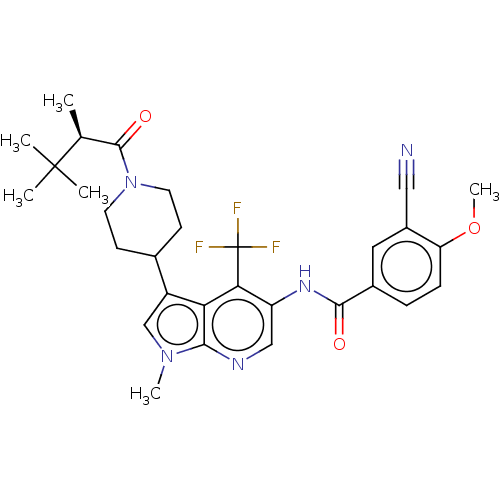
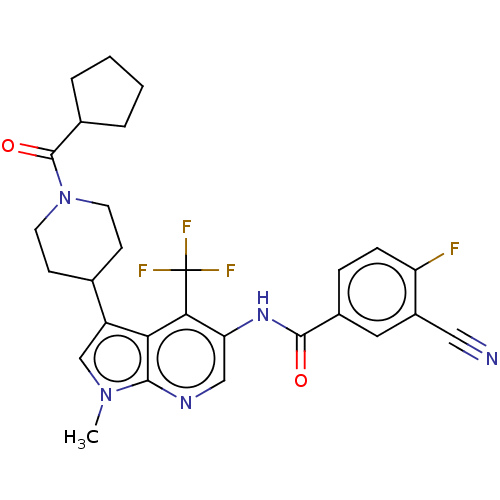
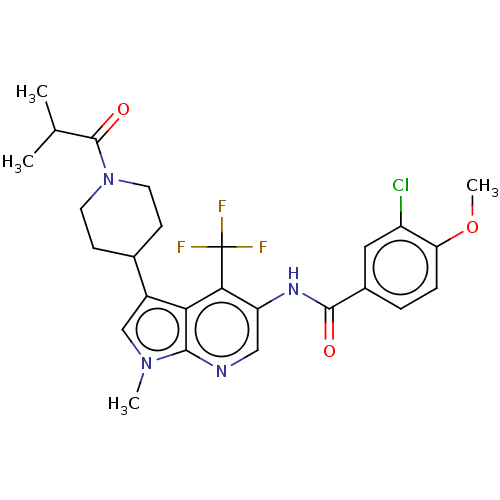
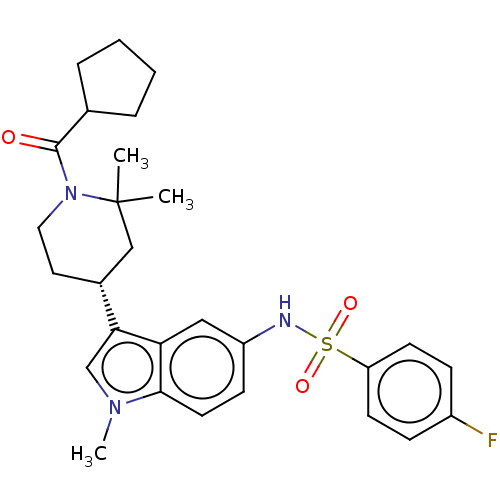
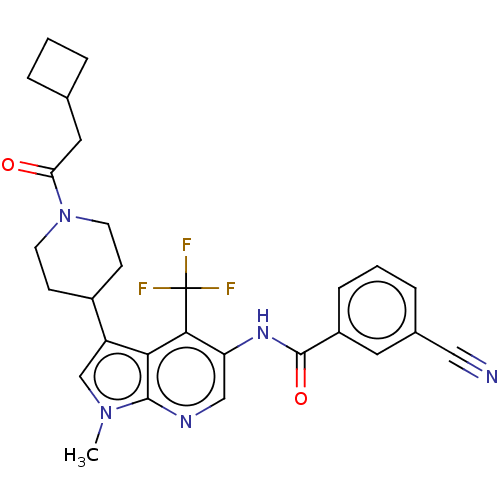
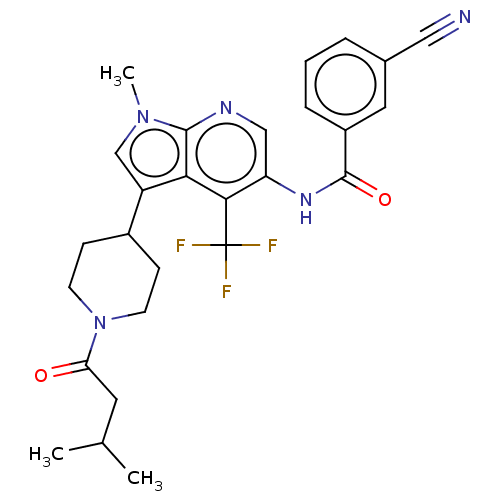
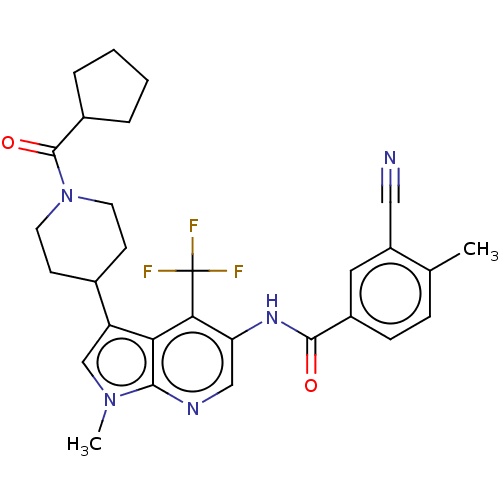
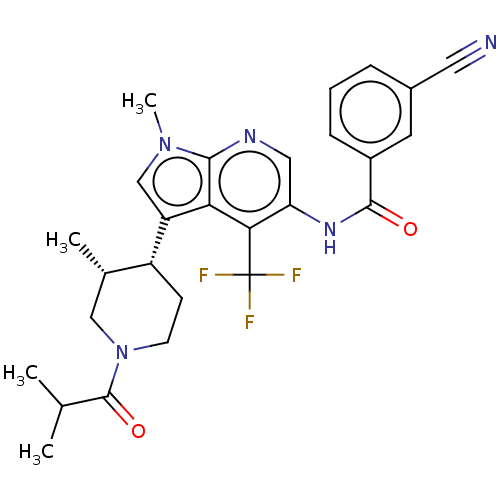
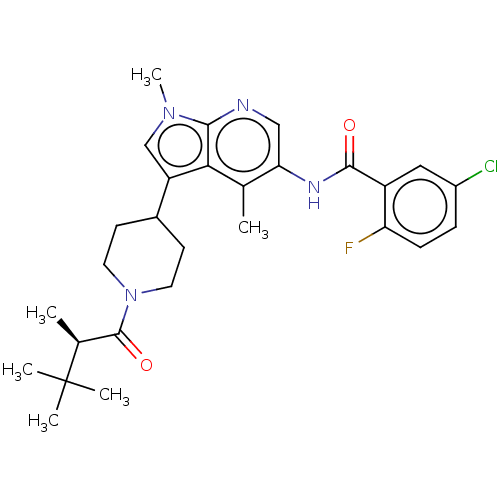
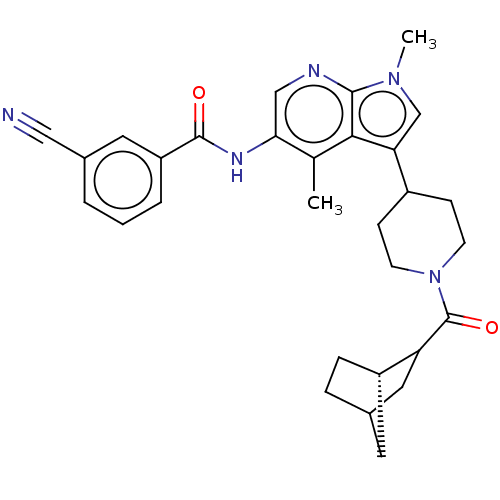
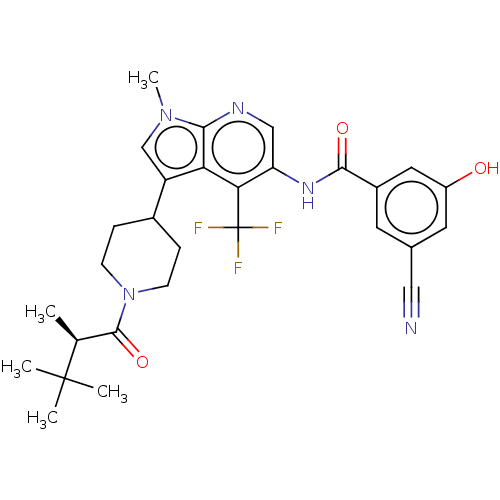
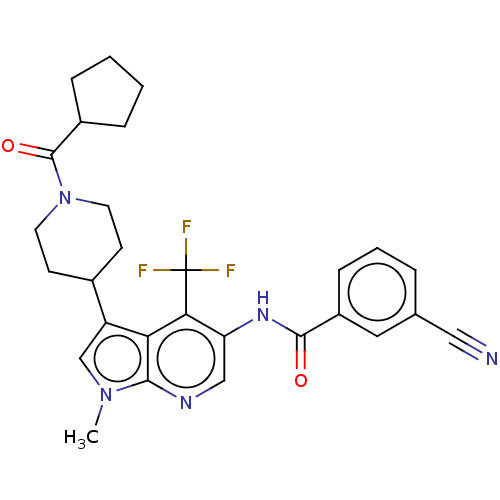
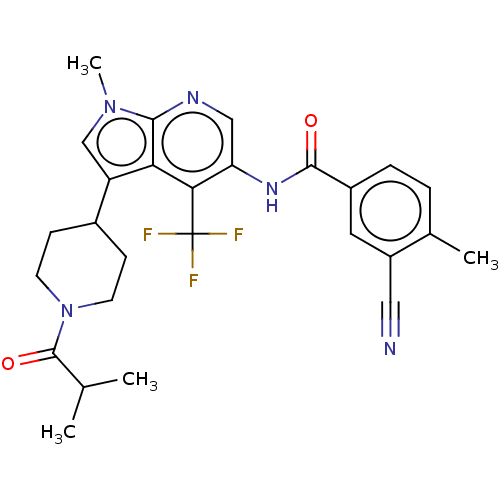
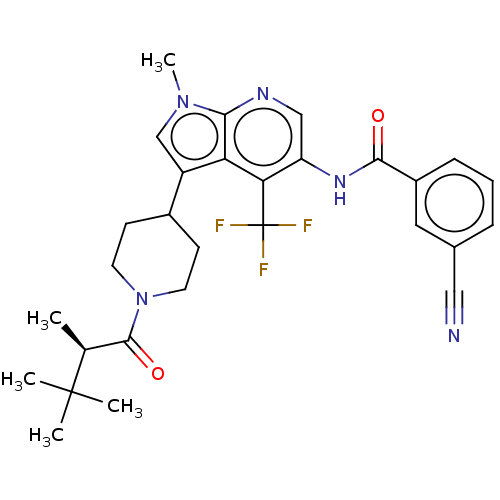
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair