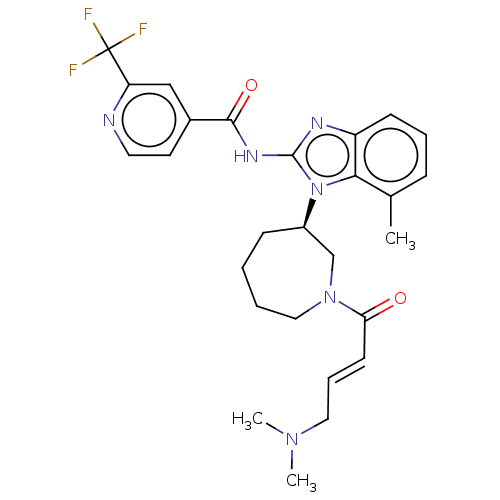
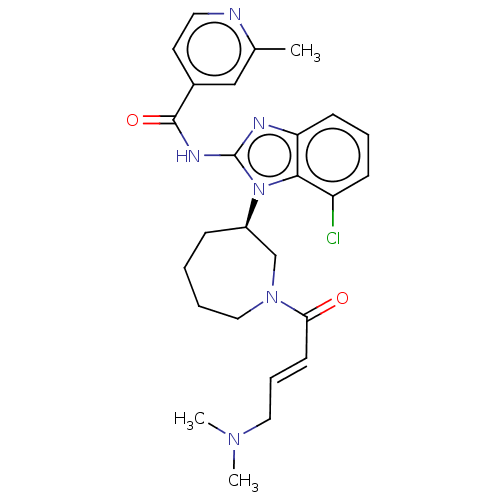
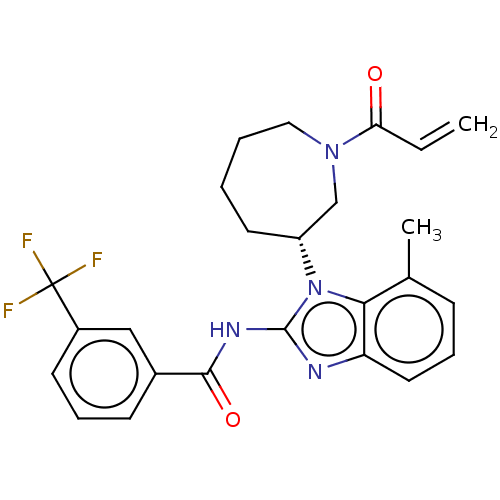
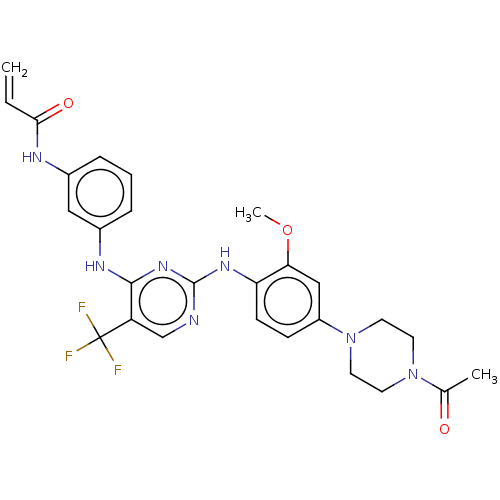
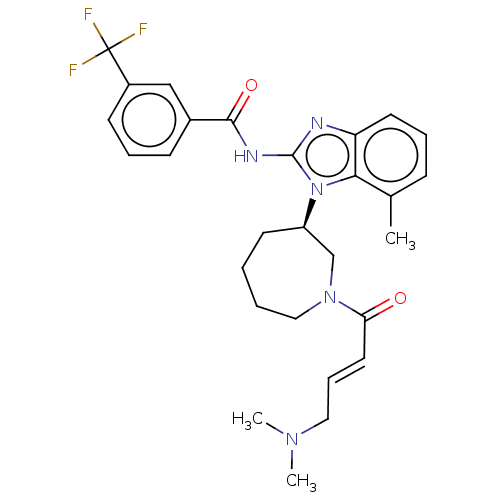
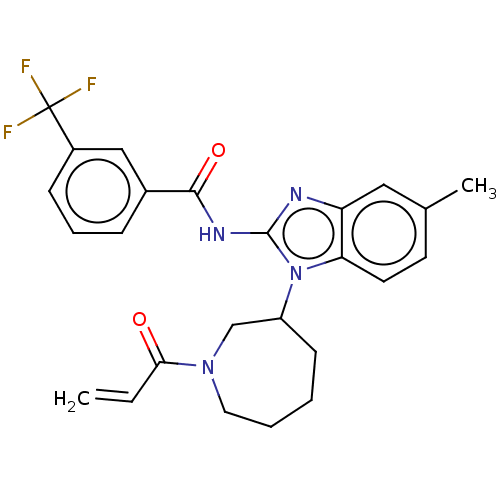
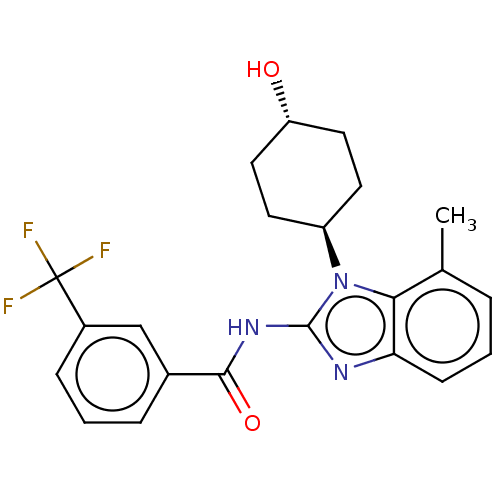
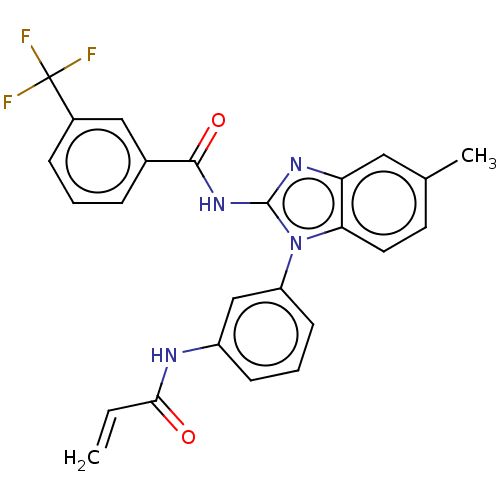
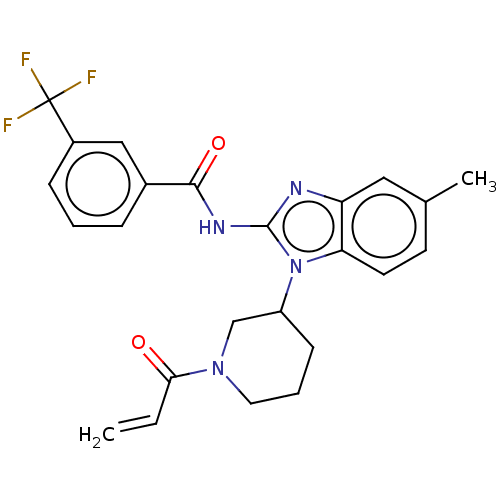
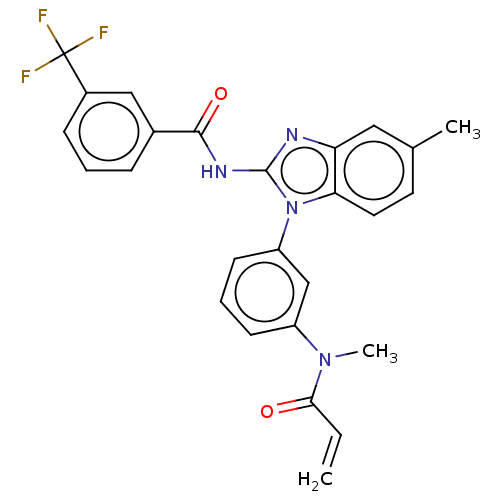
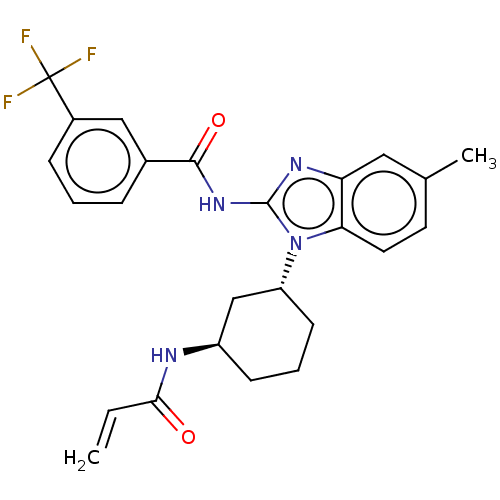
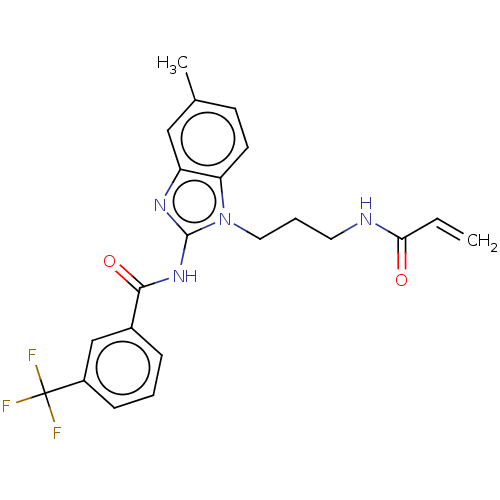
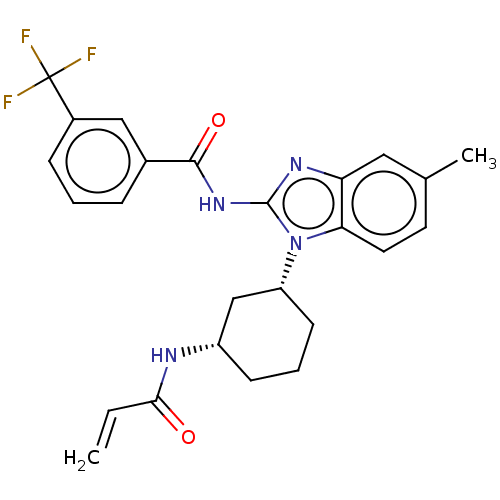
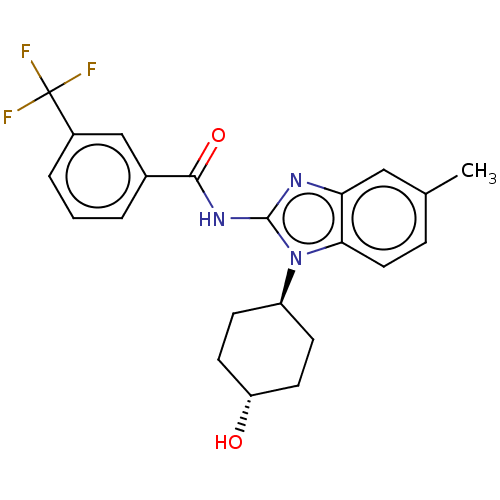
Affinity DataKi: 5nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
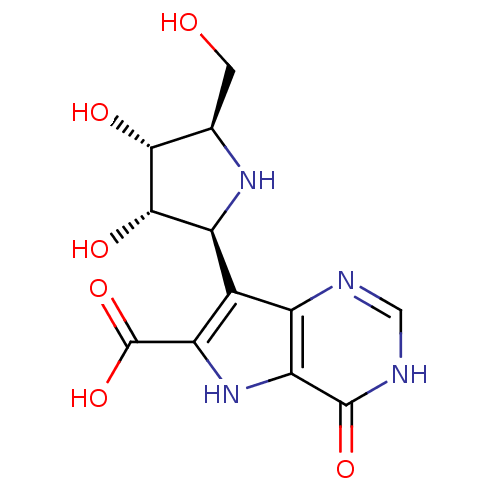
Affinity DataKi: 236nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
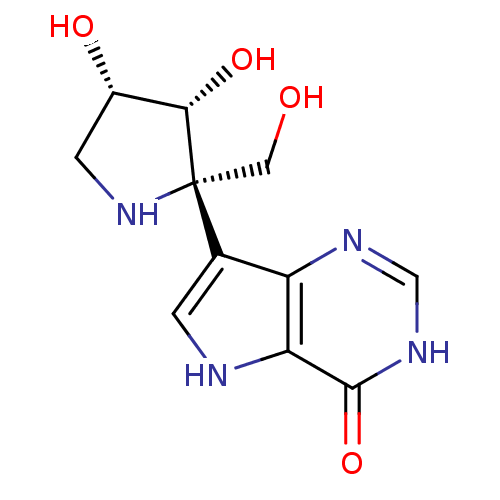
Affinity DataKi: 1.05E+3nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
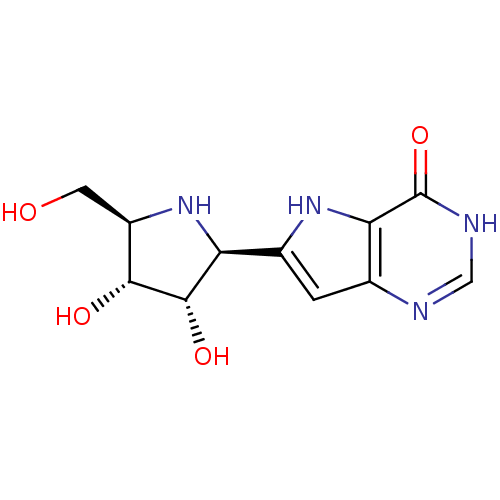
Affinity DataKi: 8.03E+3nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.34E+4nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.35E+4nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.43E+4nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataKi: 7.60E+4nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+5nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of human thymidine phosphorylase by continuous spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+5nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataKi: >2.40E+6nMAssay Description:Binding affinity towards recombinant thymidine phosphorylase TPMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibitory concentration against human thymidine phosphorylase TPMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human purine nucleoside phosphorylaseMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 161nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 262nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 351nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 4.33E+3nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 5.27E+3nMAssay Description:Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo...More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type human EGFR phosphorylation expressed in mouse NIH/3T3 cells incubated for 3 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.18E+4nMAssay Description:Inhibition of N-terminal 6x-HIS-tagged wild type human recombinant EGFR (696 to 1022 residues) expressed in Sf9 cells pre-incubated for 90 mins follo...More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.67E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 1.76E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.22E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair


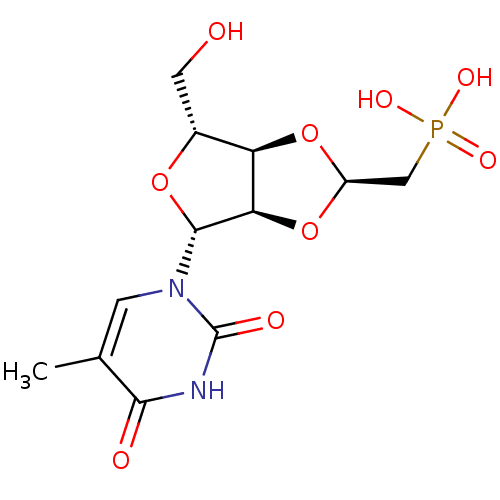
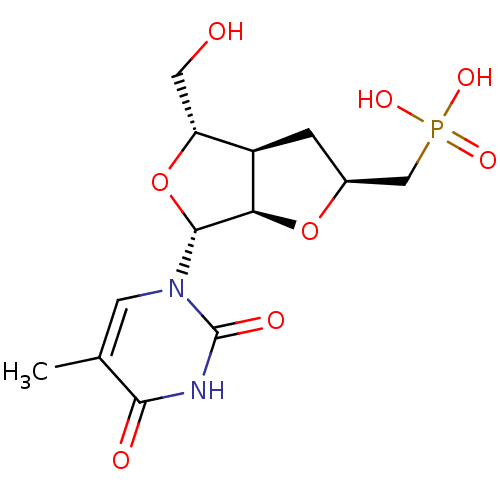
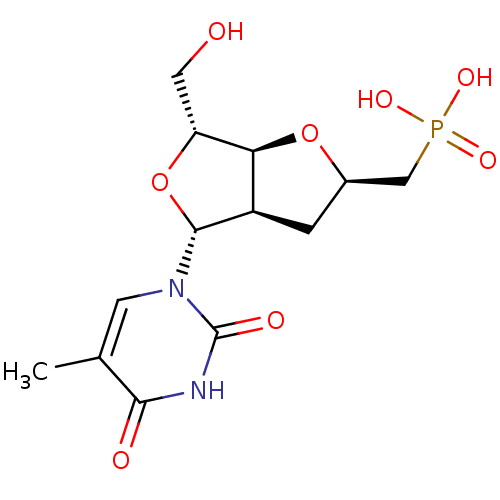

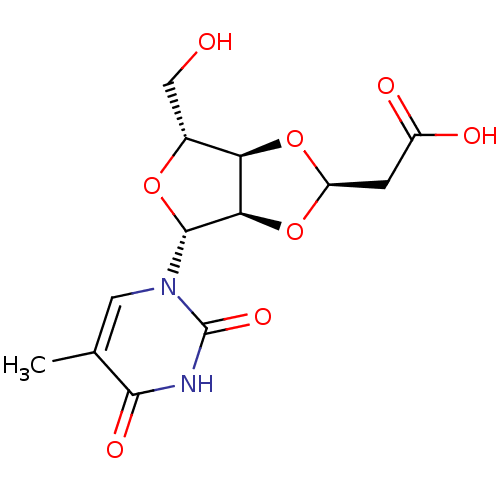


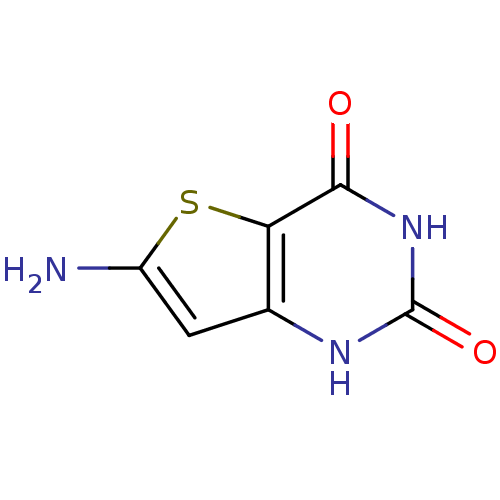
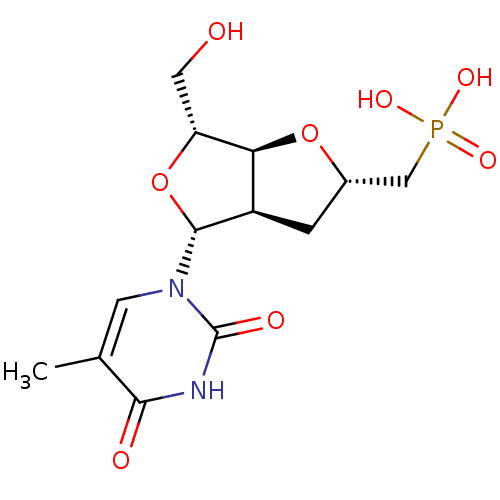
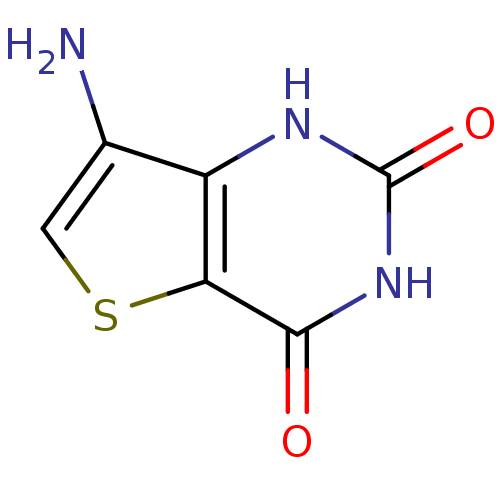
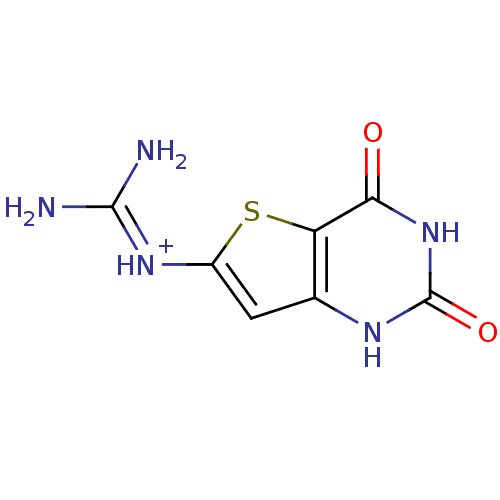
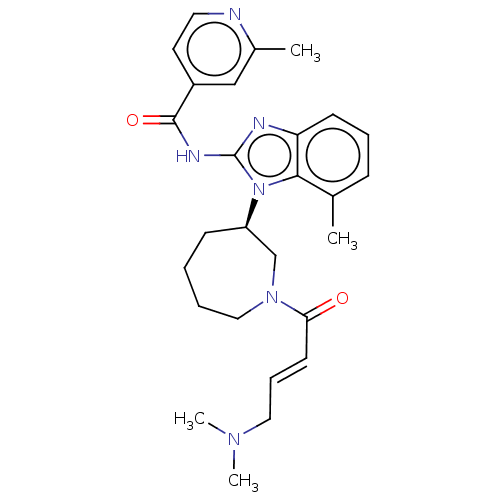
 3D Structure (crystal)
3D Structure (crystal)