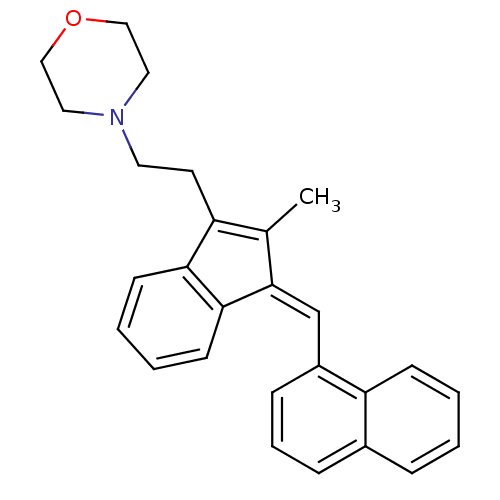
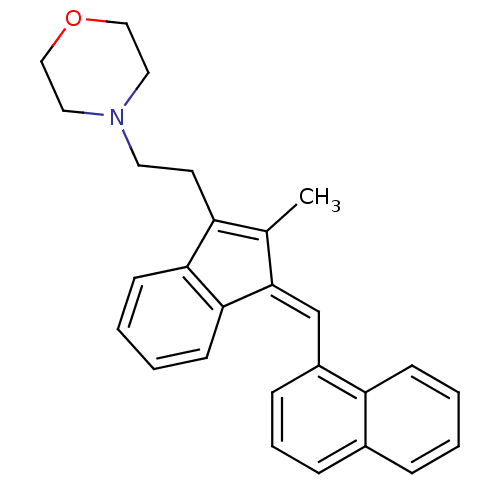
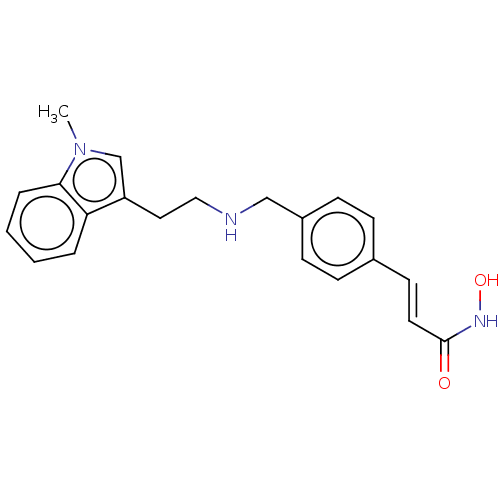
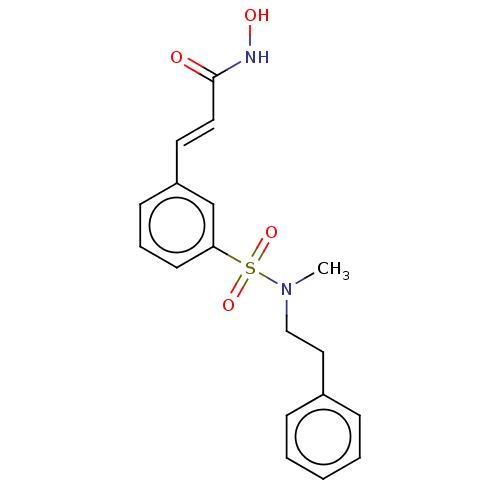
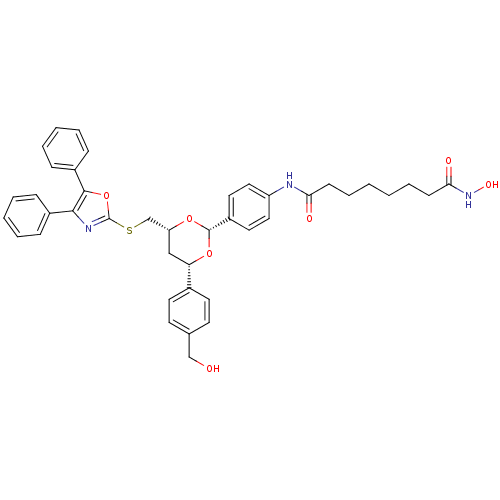
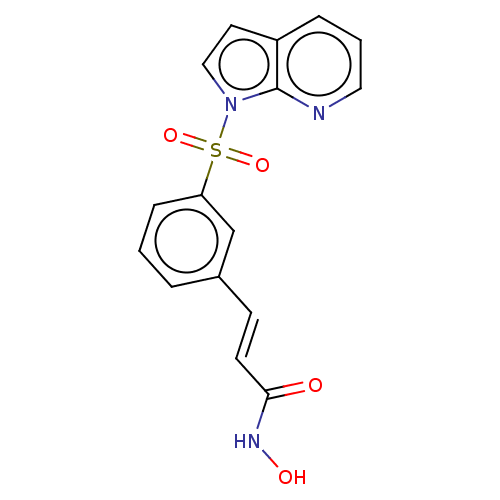
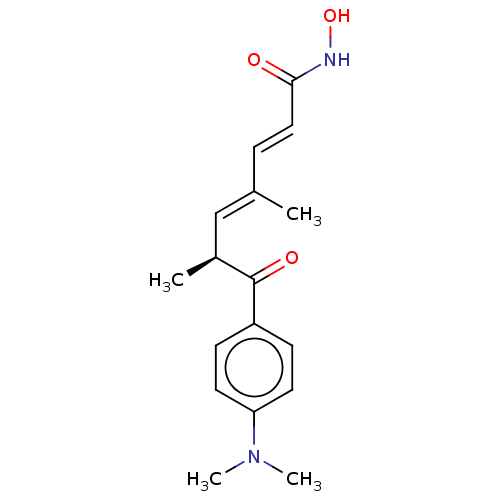
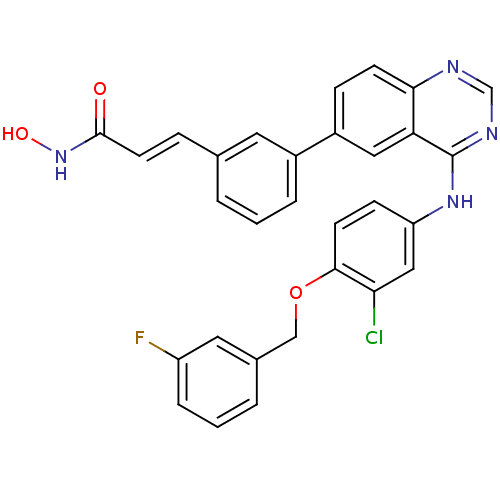
Affinity DataKi: 0.280nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinity to human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligandMore data for this Ligand-Target Pair
Affinity DataKi: 9.44nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligandMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand at site 1More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 94nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 132nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 148nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 527nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 611nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand at site 2More data for this Ligand-Target Pair
Affinity DataKi: 658nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 928nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligandMore data for this Ligand-Target Pair
Affinity DataKi: 1.22E+3nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.95E+3nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligandMore data for this Ligand-Target Pair
Affinity DataKi: 2.18E+3nMAssay Description:Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligandMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
In DepthDetails
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant HDAC3 using fluorogenic HDAC substrate measured after 10 mins by fluorimetry assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant HDAC2 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 22nMAssay Description:Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate measured after 30 mins by fluorimetry assayMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate measured after 45 mins by fluorimetry assayMore data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Negative allosteric modulation of rat mGluR5 receptor expressed in HEK293 cells assessed as intracellular calcium flux after 170 seconds by FLIPR ass...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)