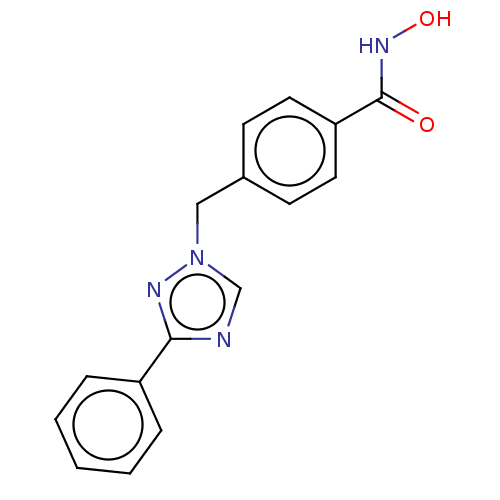
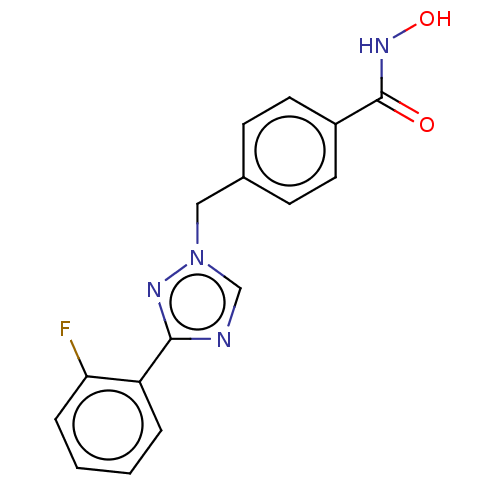
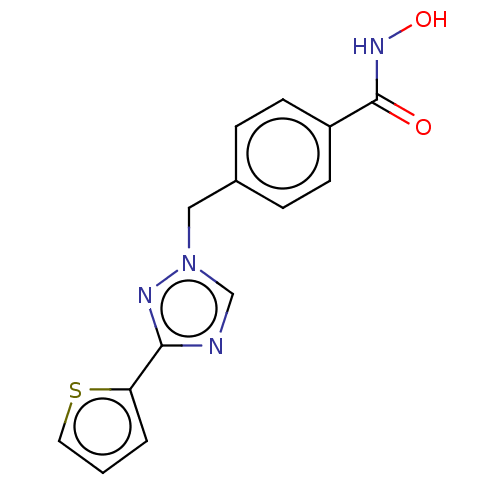
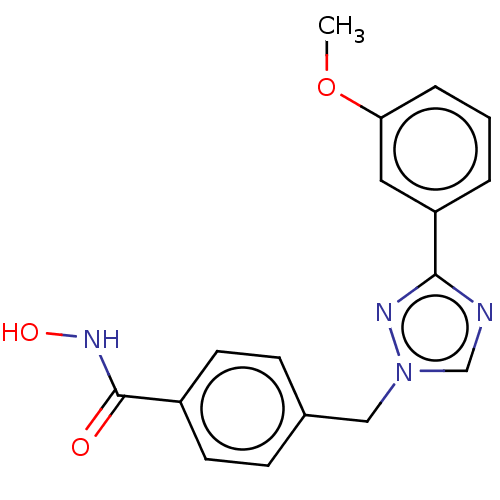
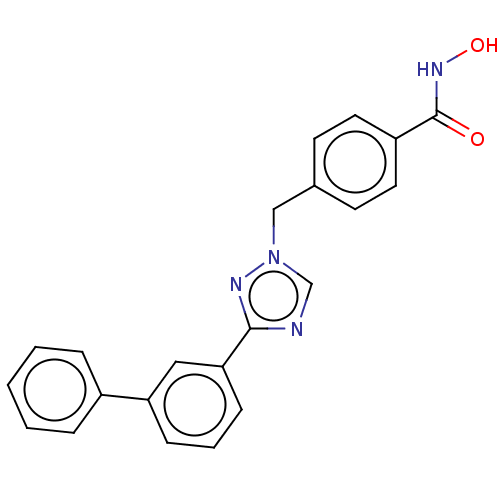
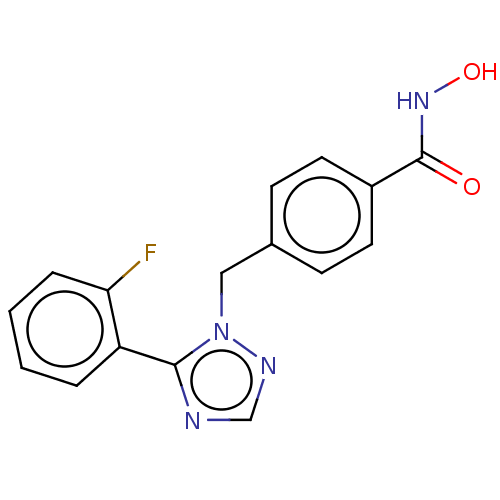
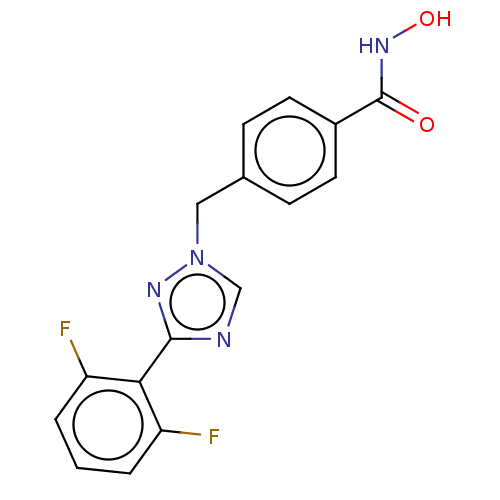
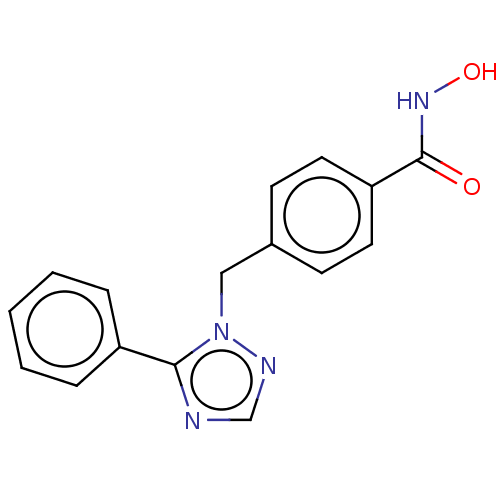
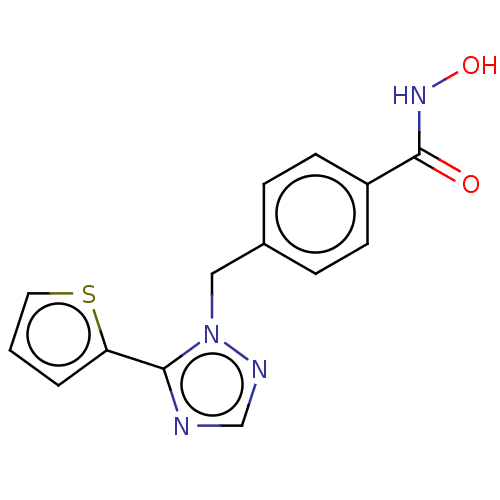
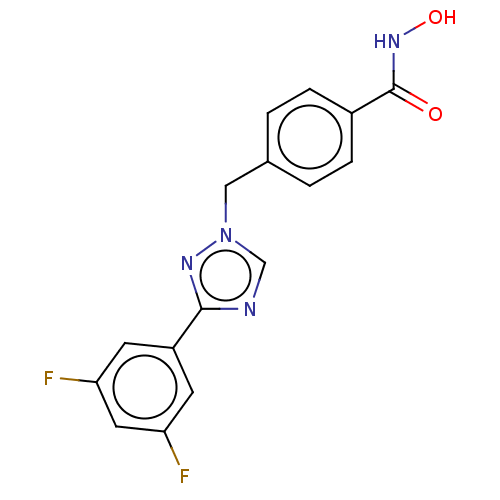
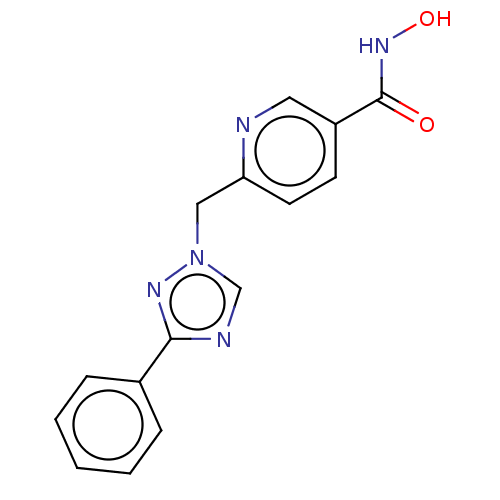
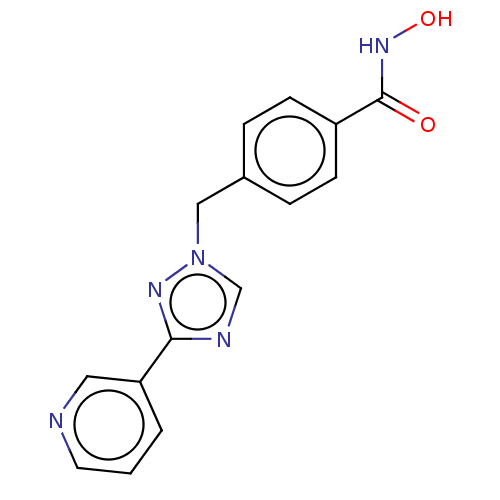
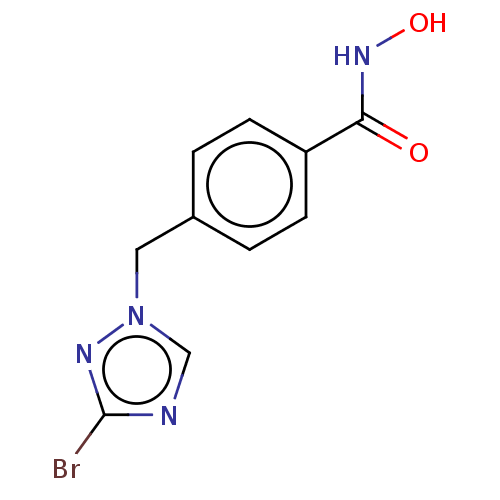
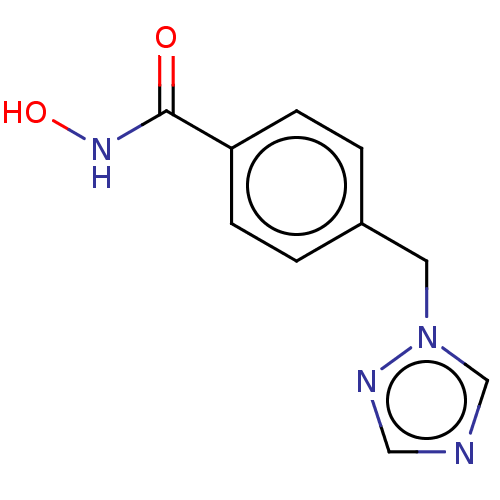
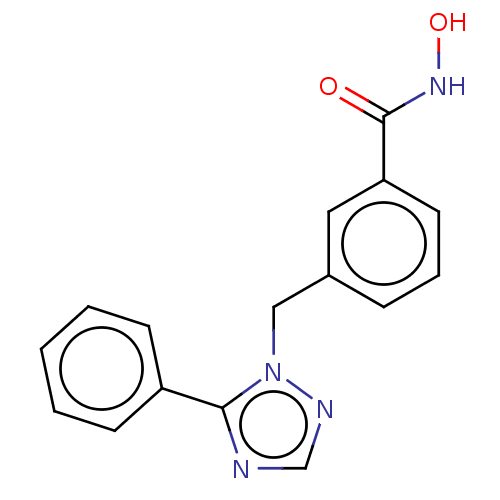
Affinity DataKi: 110nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataKi: 1.35E+3nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataKi: 2.73E+3nMAssay Description:Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 4.60nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 6.40nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 7.10nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 7.10nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 8.70nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 8.90nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 12nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 14nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 15nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 17nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 18nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 21nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 28nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 44nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 71nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by fluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cellsMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 111nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 122nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 132nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 132nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 152nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 164nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 167nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 168nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 170nMAssay Description:Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by fluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: 197nMAssay Description:Inhibitory activity against acetylcholinetransferase (AChE) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 206nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 212nMAssay Description:Inhibition of choline acetyltransferase (CAT) enzymeMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails

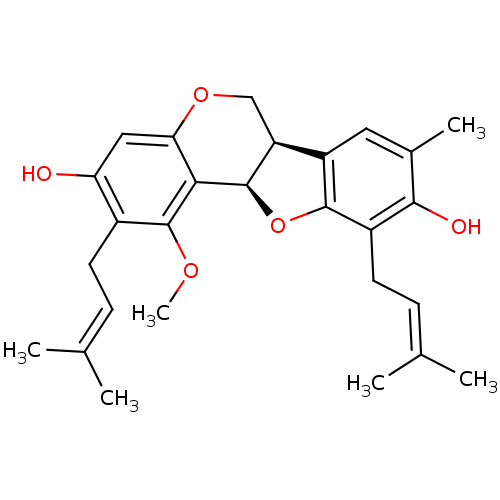
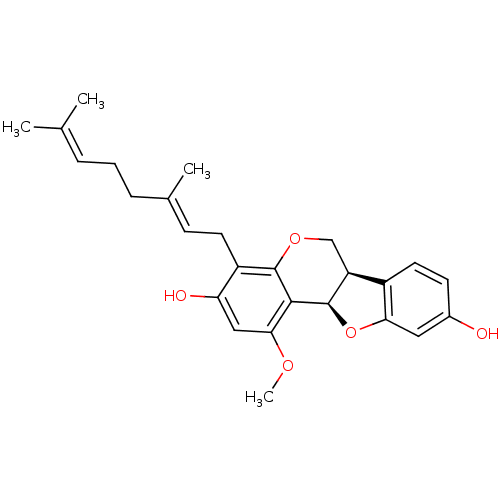
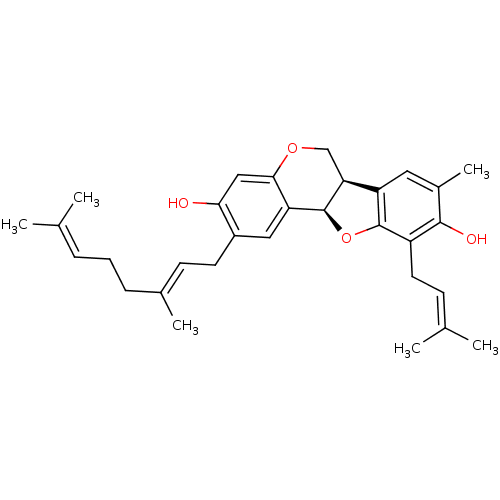
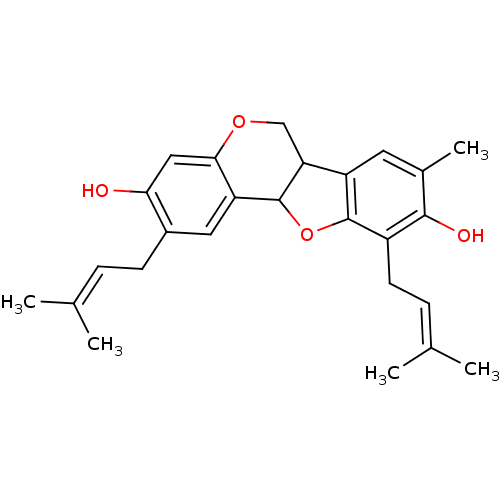


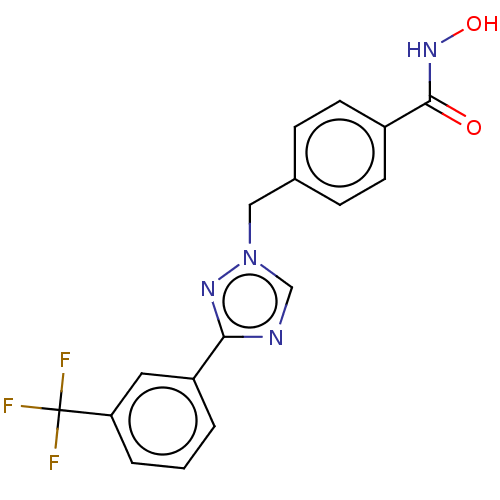
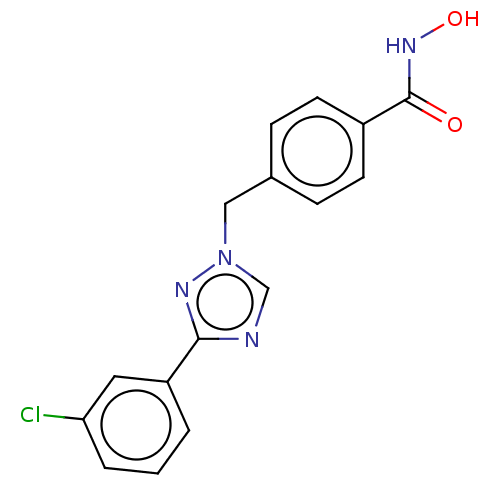
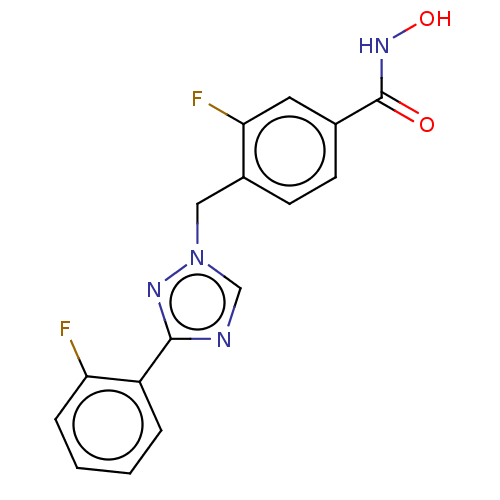
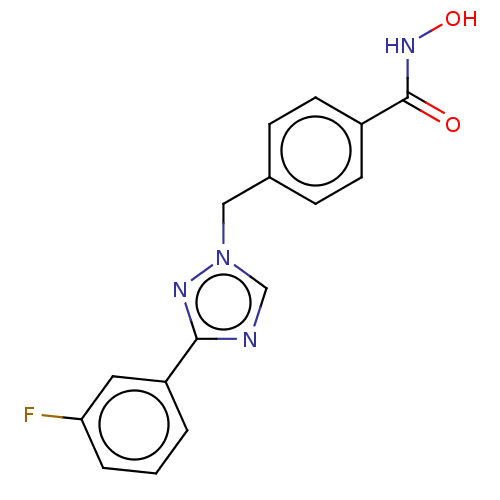
 3D Structure (crystal)
3D Structure (crystal)