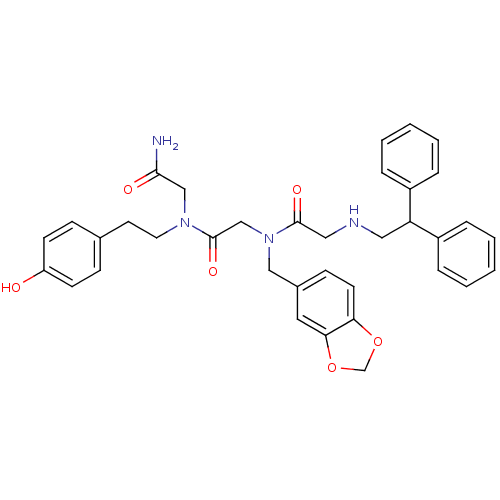
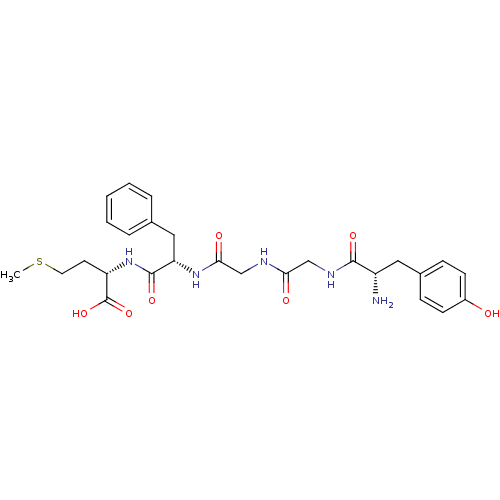
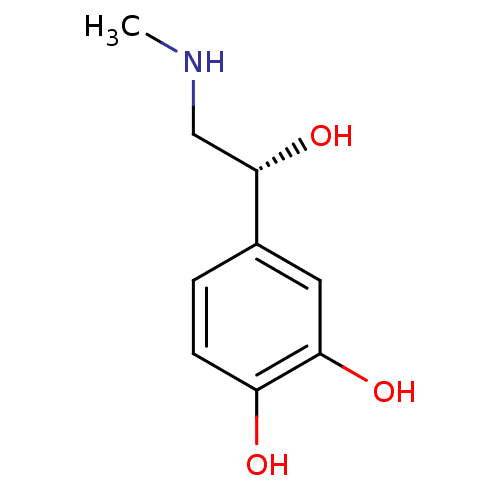
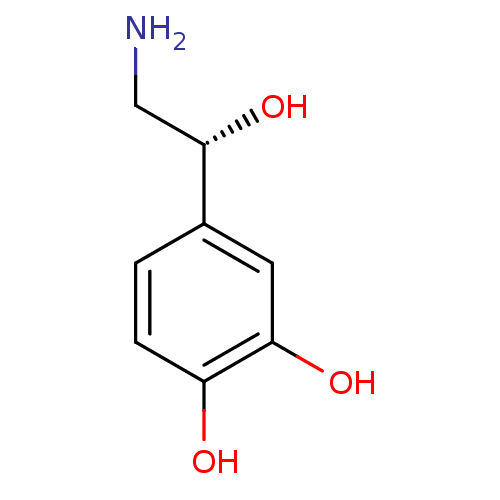
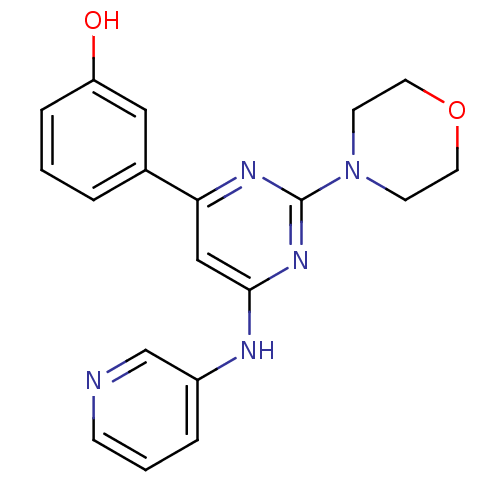
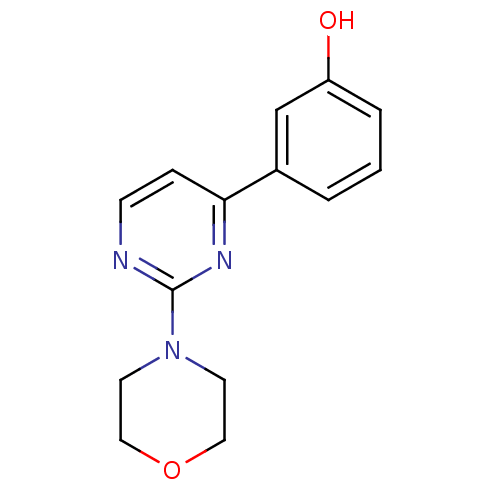
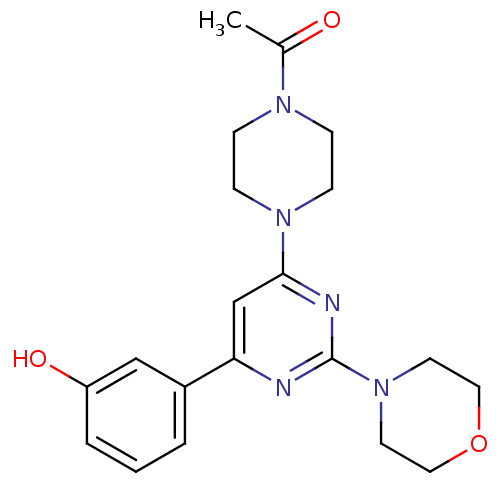
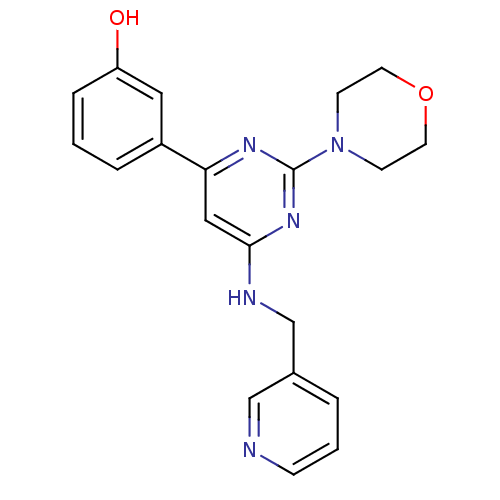
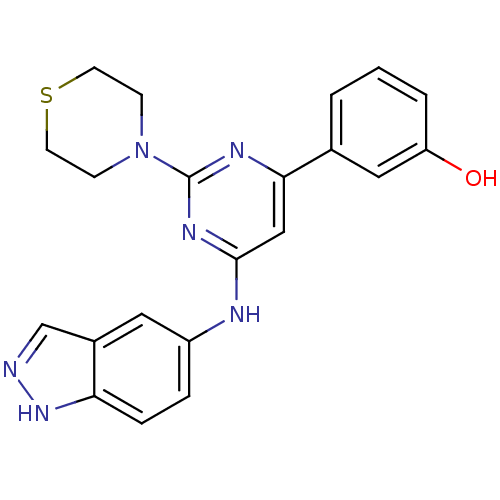
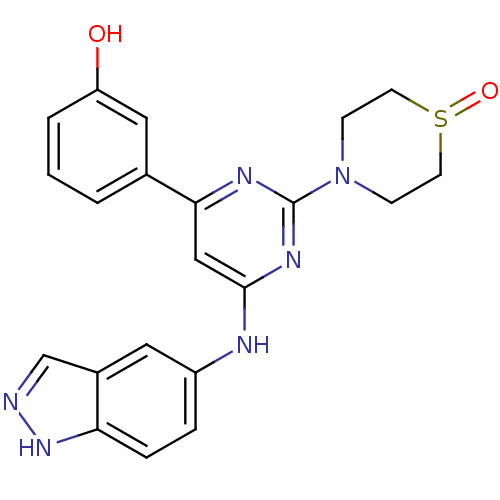
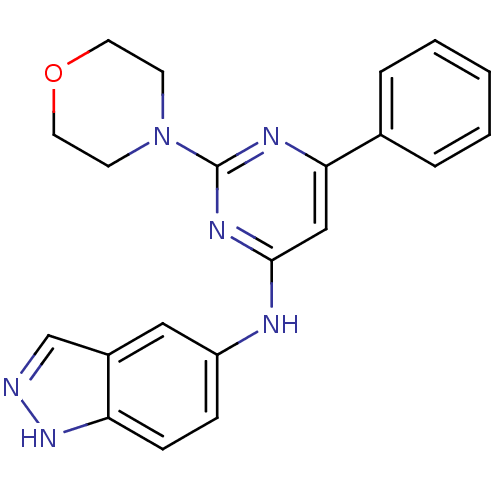
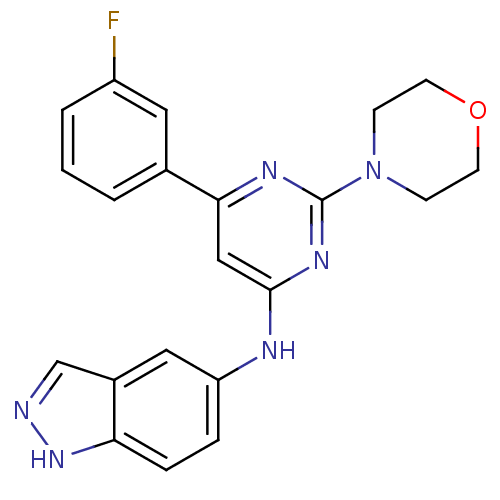
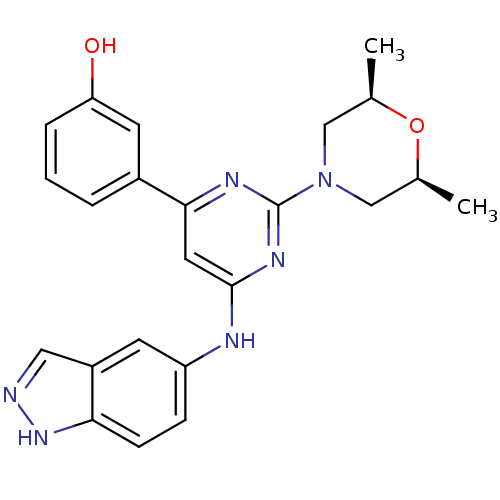
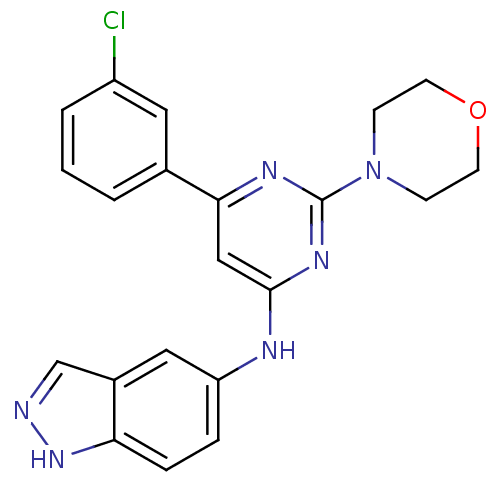
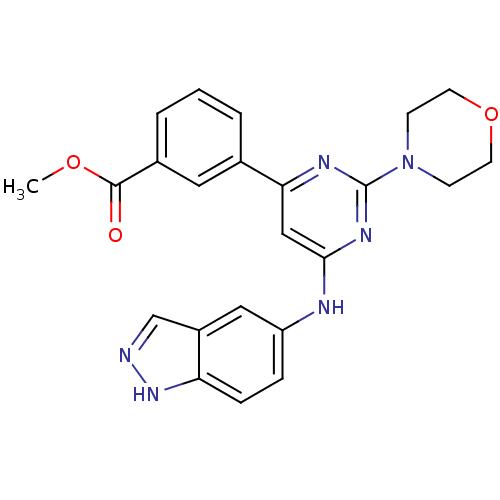
Affinity DataKi: 0.200nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGOMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGOMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGOMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid libraryMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of PI3KbetaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of PI3KbetaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 570nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 570nMAssay Description:Inhibition of PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 910nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of PI3KalphaMore data for this Ligand-Target Pair
Target3-phosphoinositide-dependent protein kinase 1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of PDK1More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)