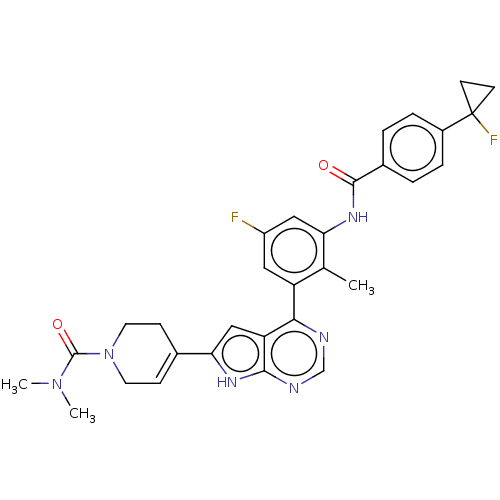
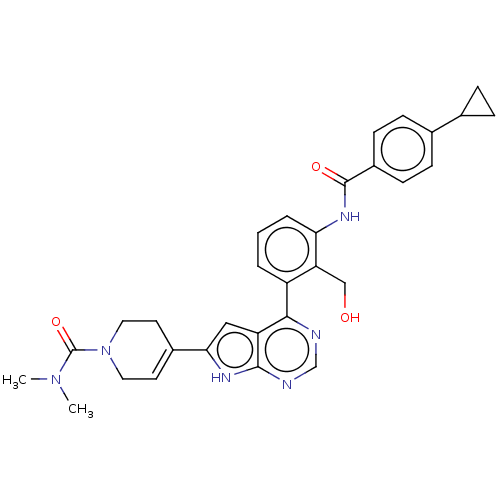
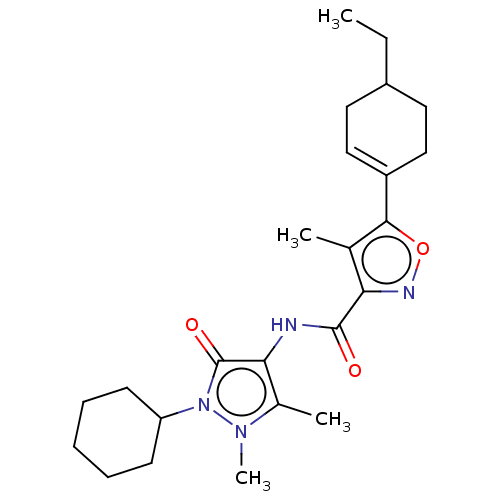
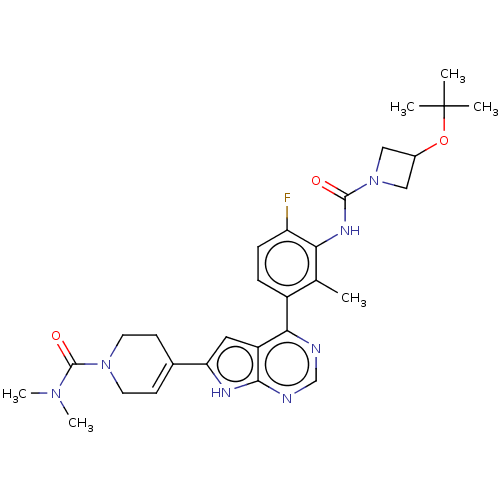
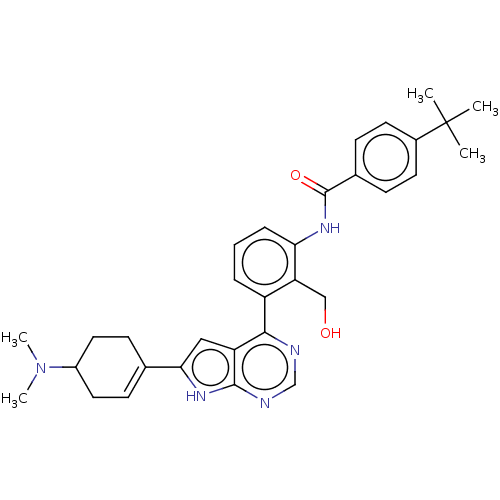
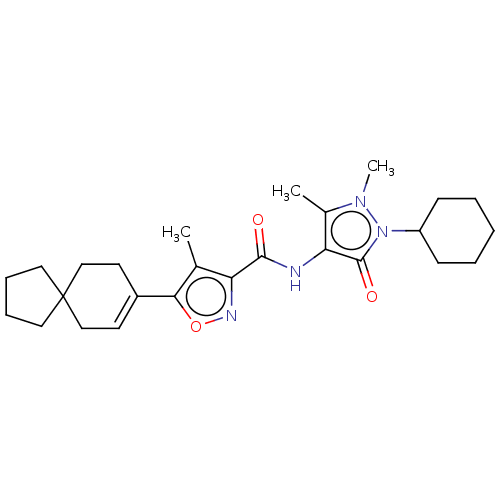
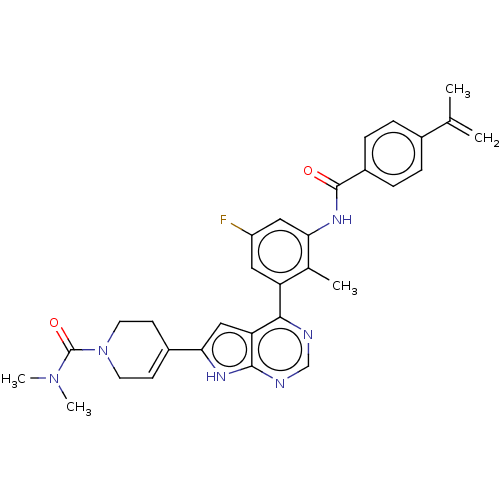
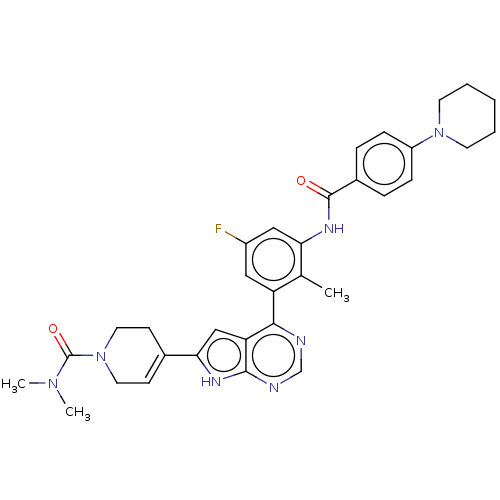
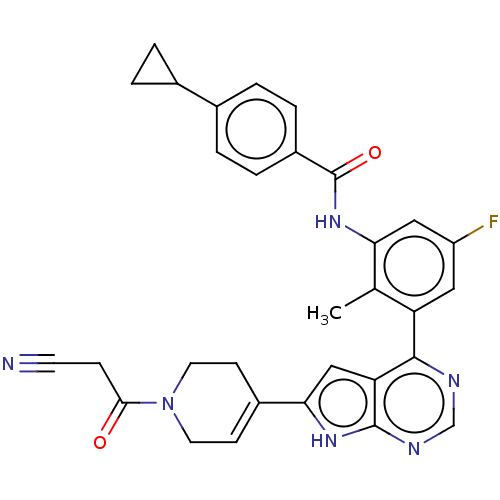
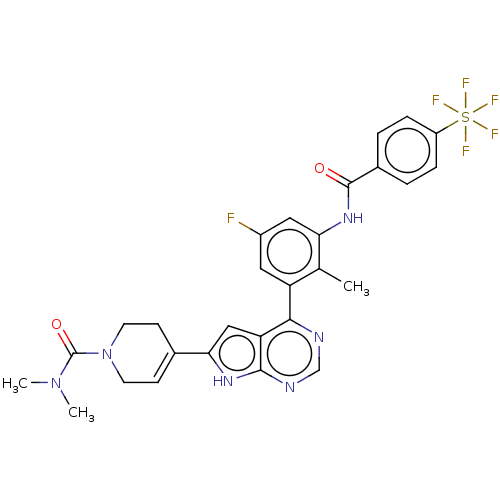
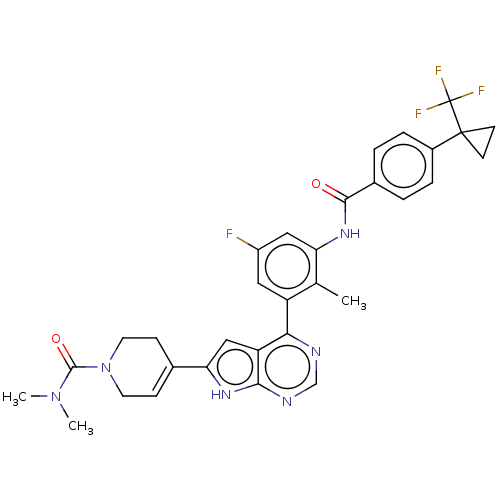
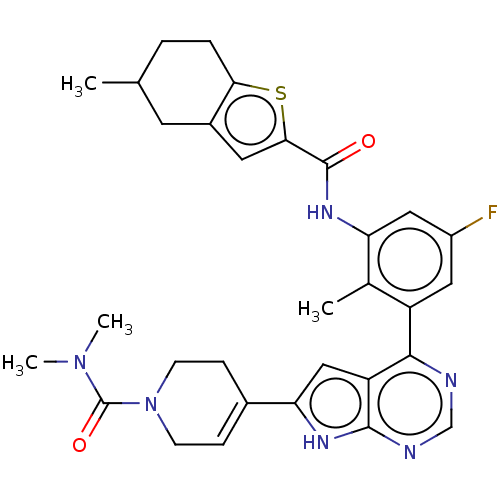
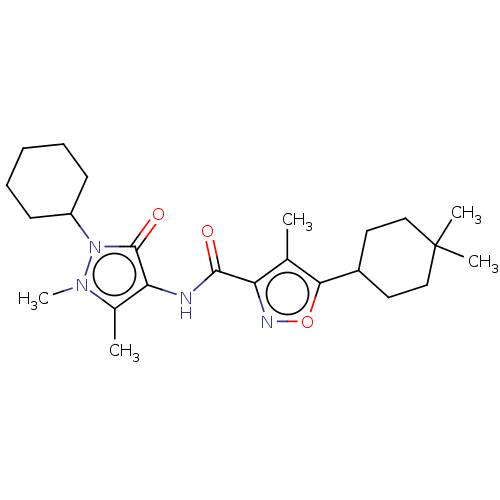
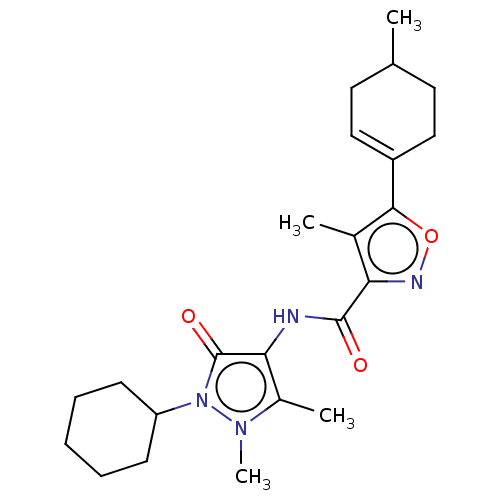
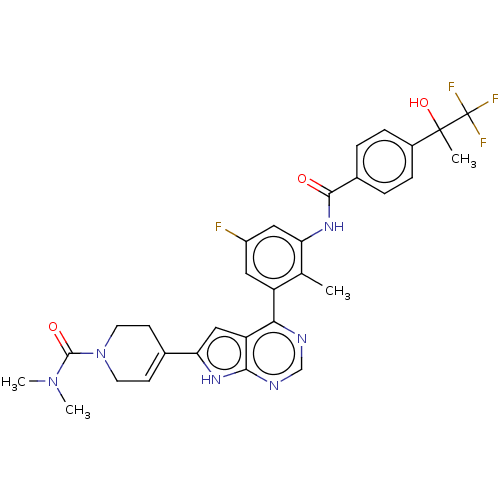
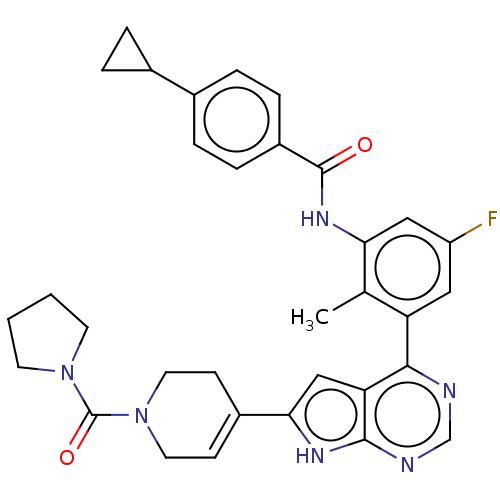
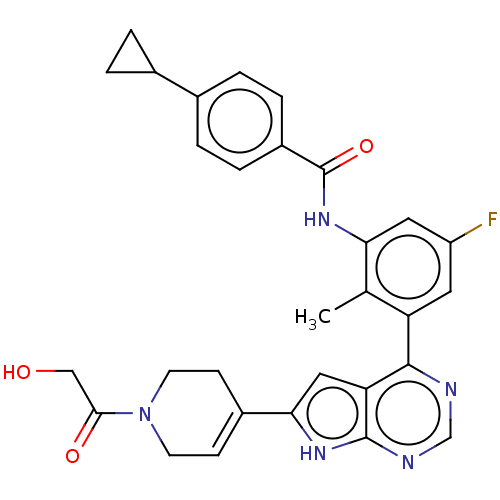
Affinity DataIC50: 0.900nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMT: 2°CAssay Description:For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare...More data for this Ligand-Target Pair