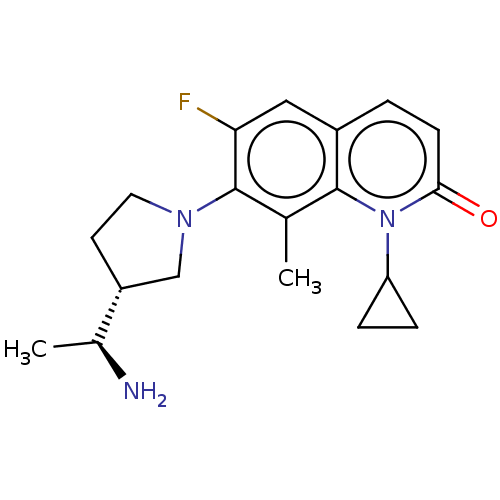
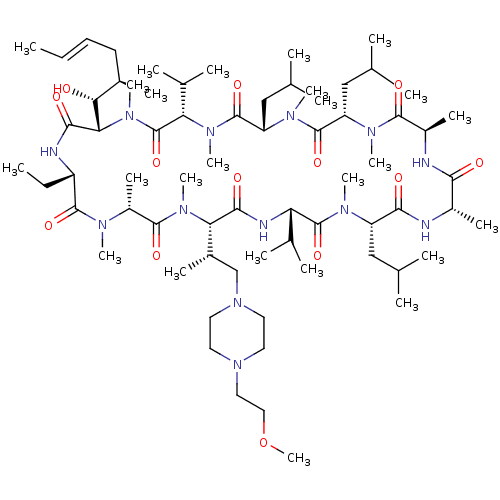
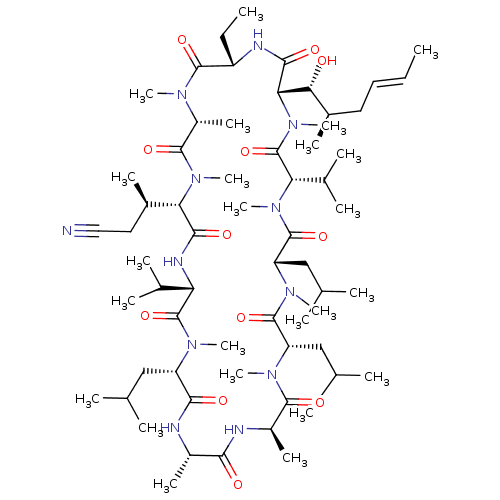
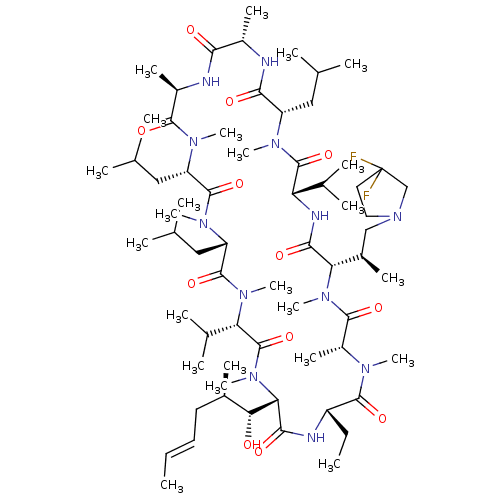
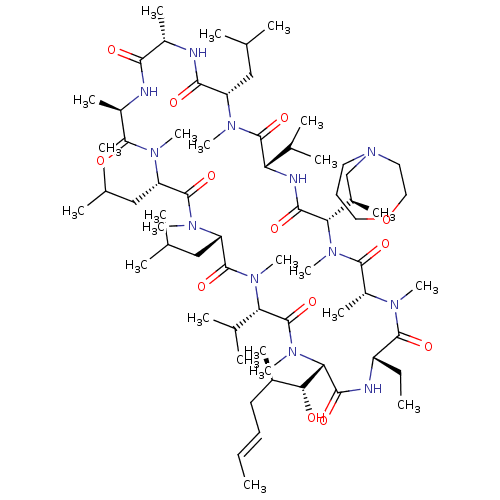
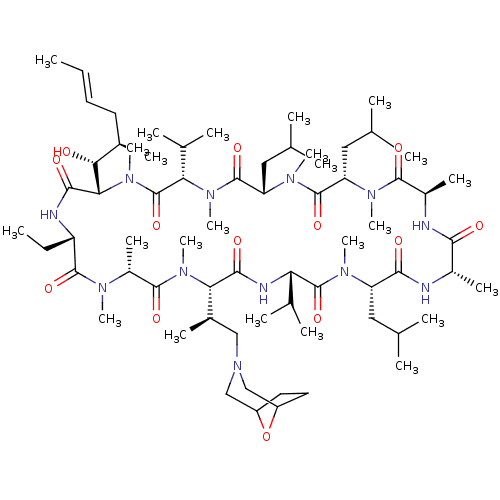
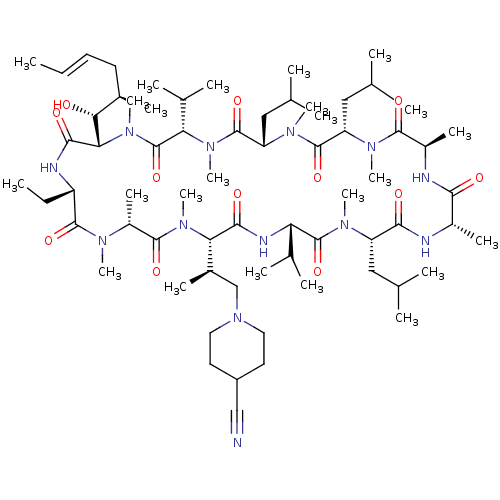
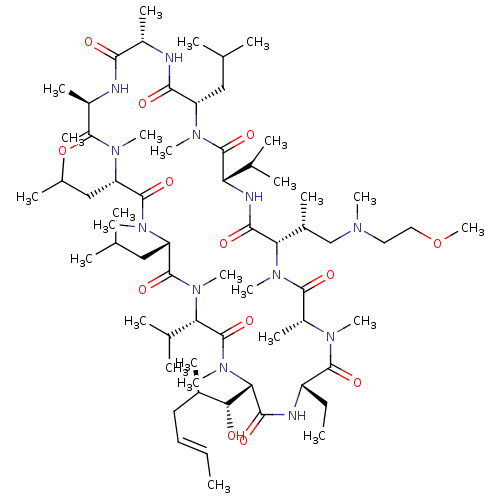
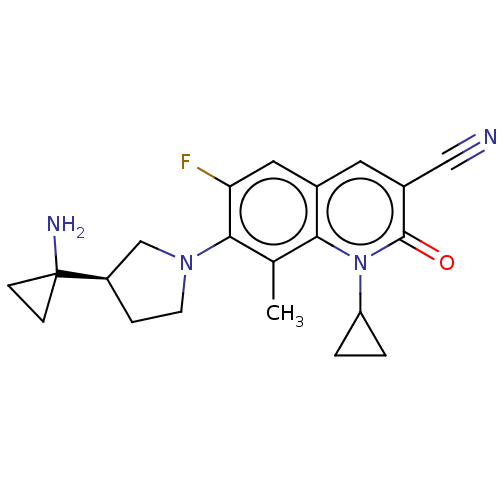
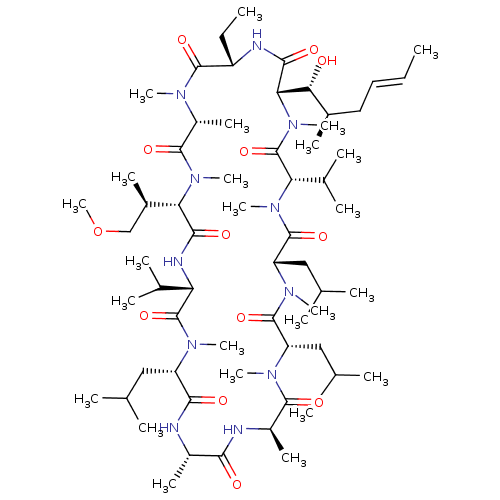
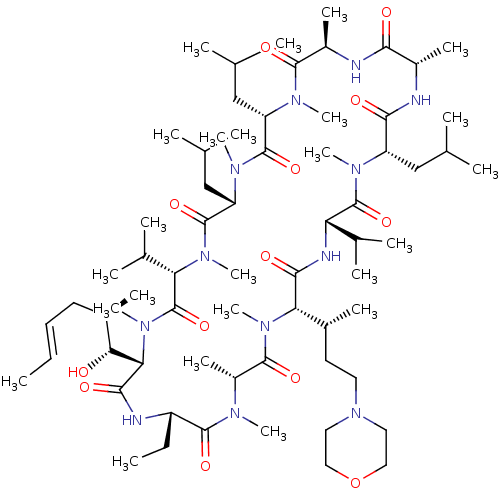
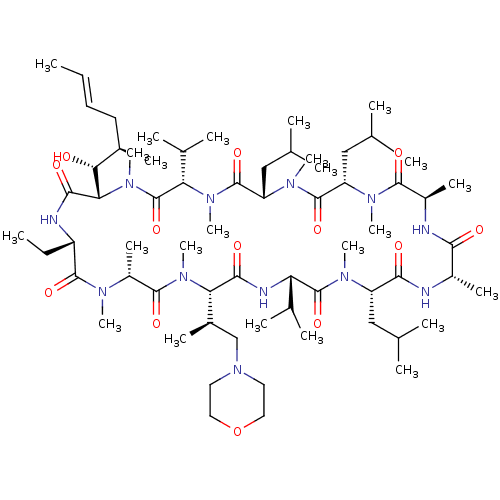
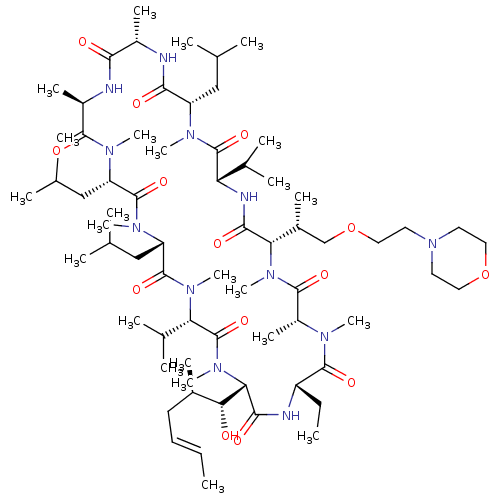
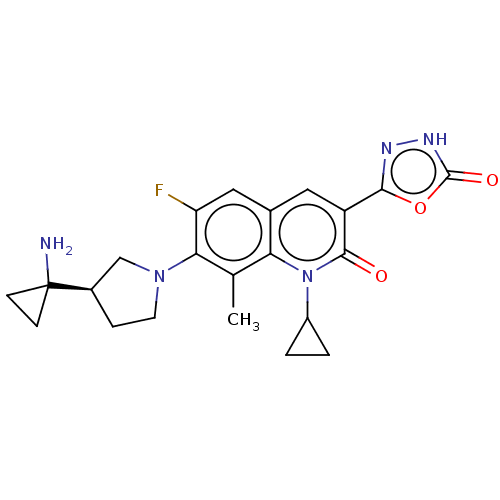
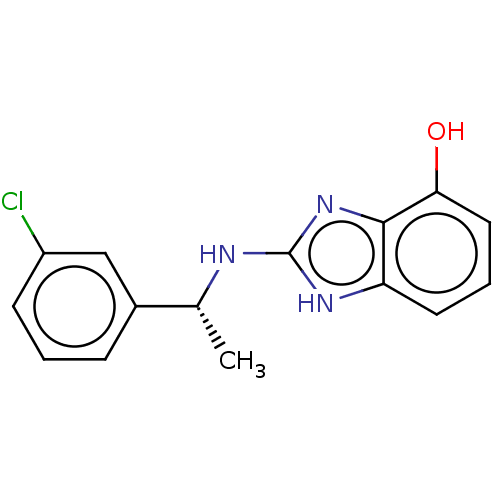
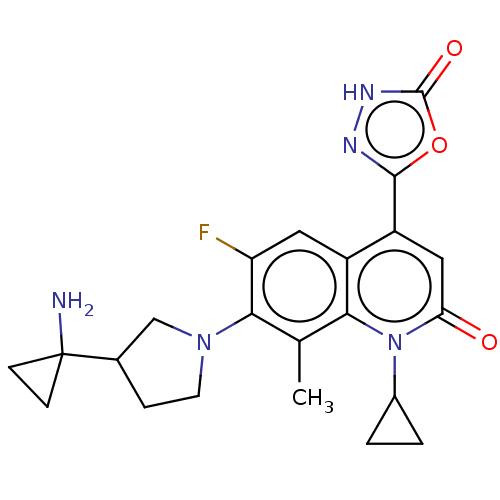
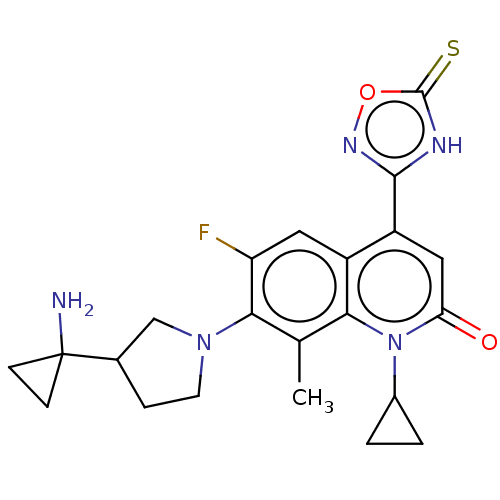
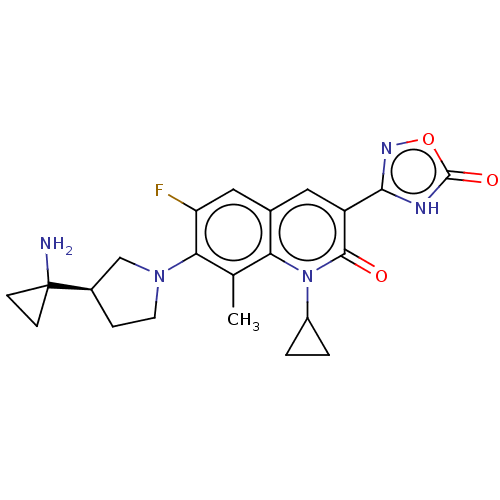
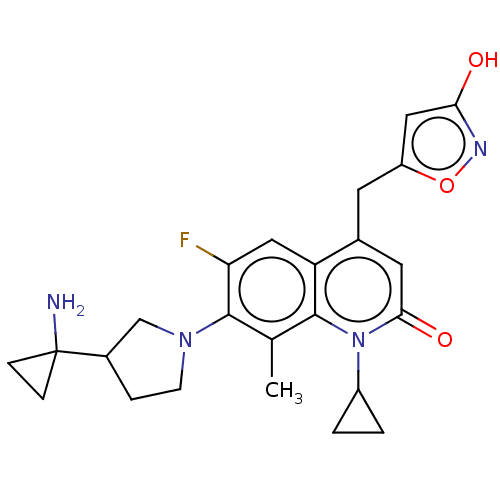
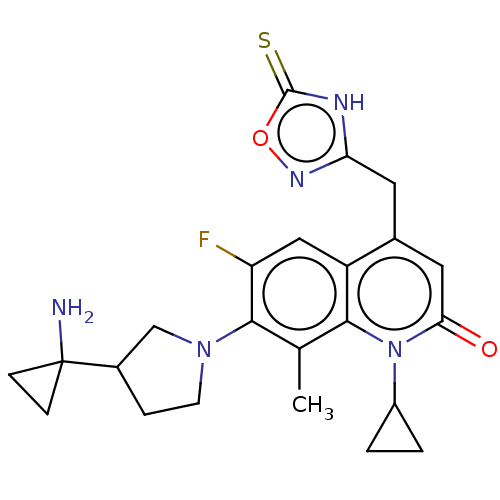
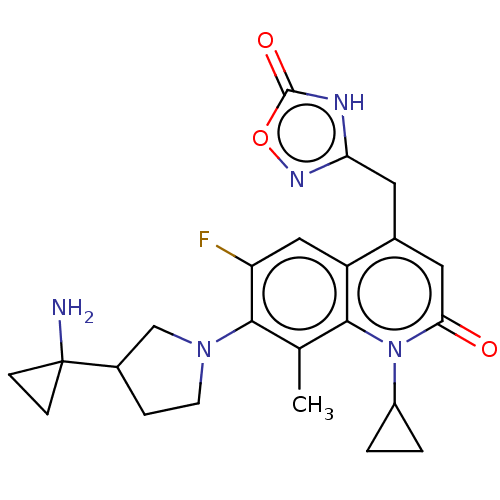
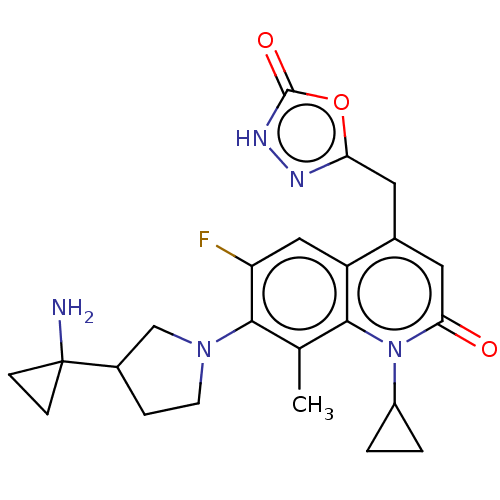
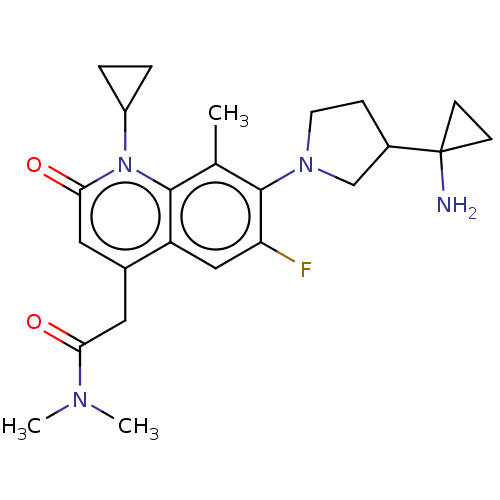
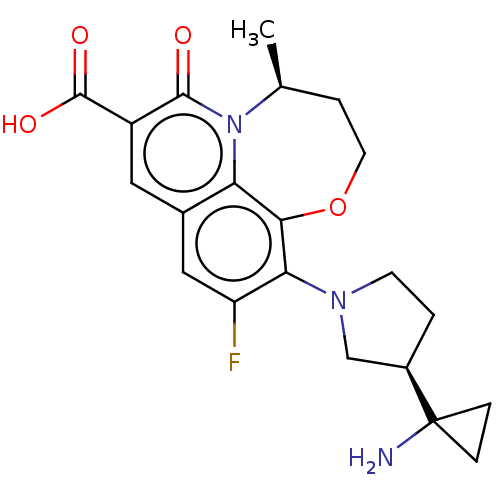
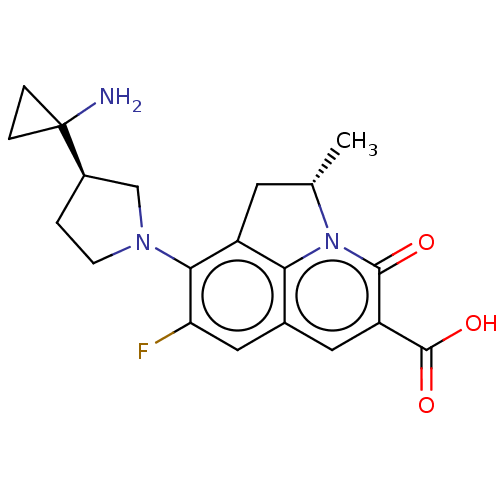
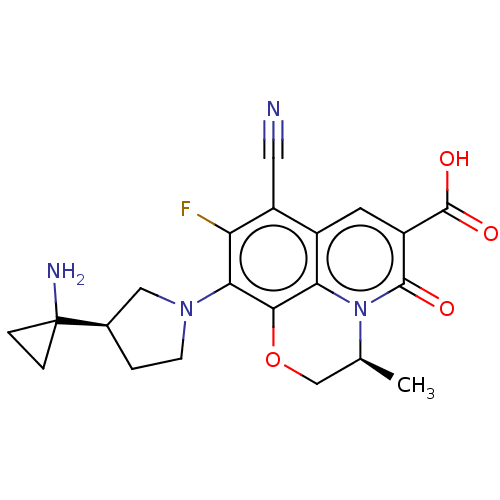
TargetBile salt export pump(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei...More data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei...More data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.25E+3nMAssay Description:Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetBile salt export pump(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.91E+3nMAssay Description:Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetBifunctional coenzyme A synthase(Homo sapiens)
Novartis Institutes for BioMedical Research , 5300 Chiron Way , Emeryville , California 94608 , United States.
Curated by ChEMBL
Novartis Institutes for BioMedical Research , 5300 Chiron Way , Emeryville , California 94608 , United States.
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human PPATMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp methodMore data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair