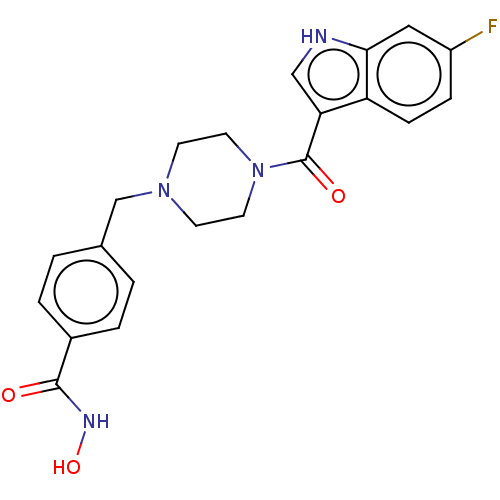
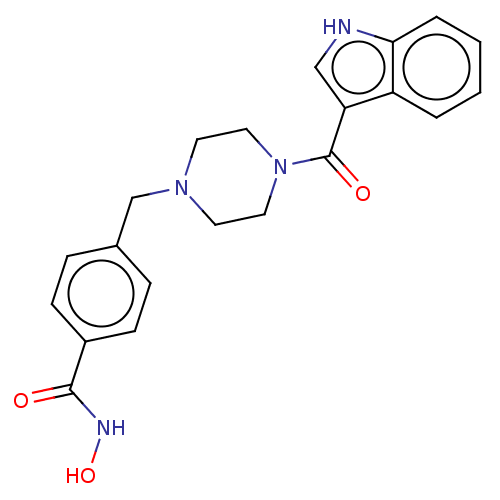
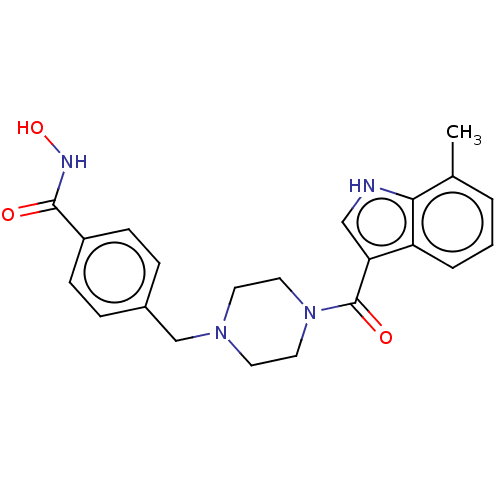
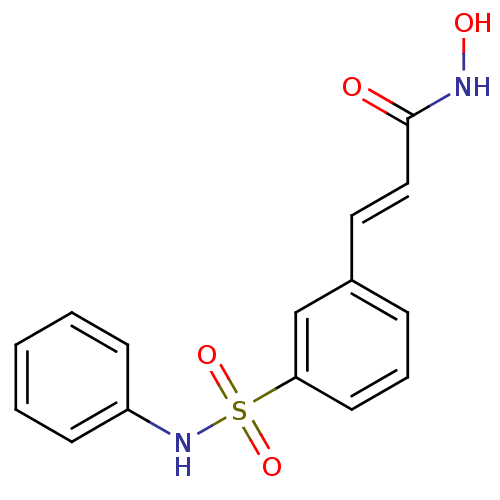
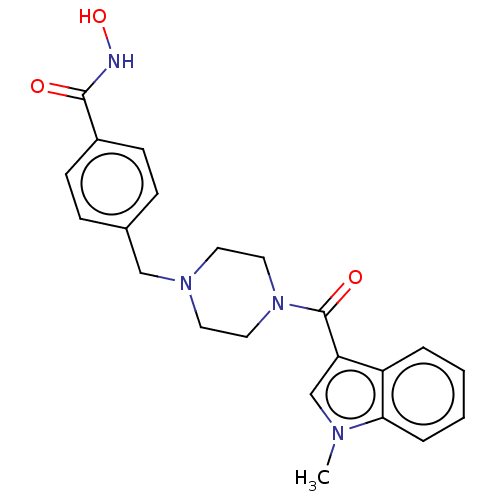
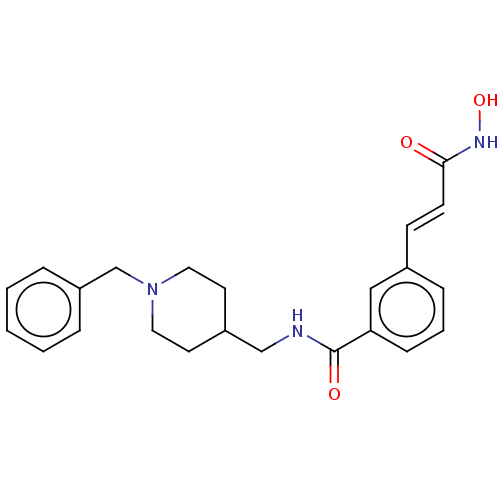
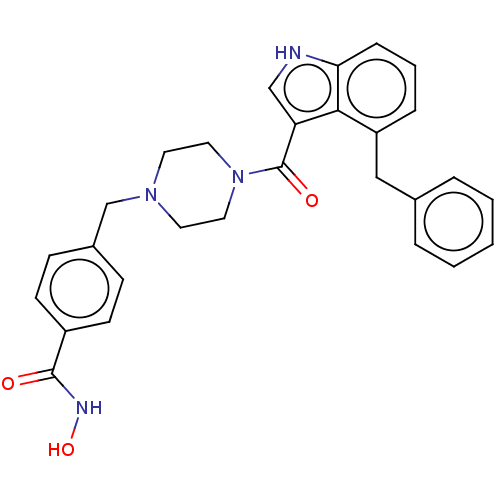
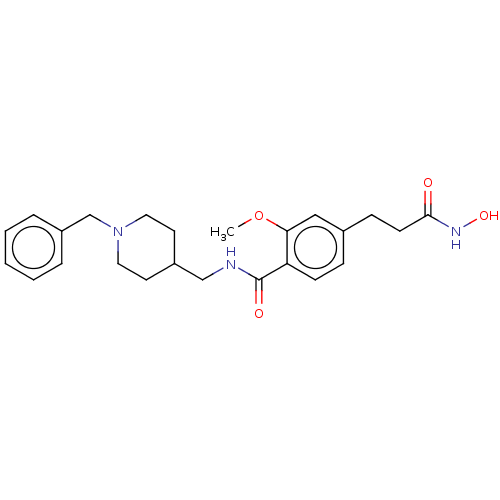
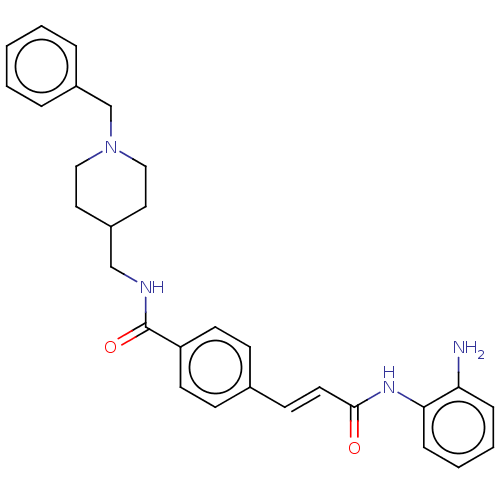
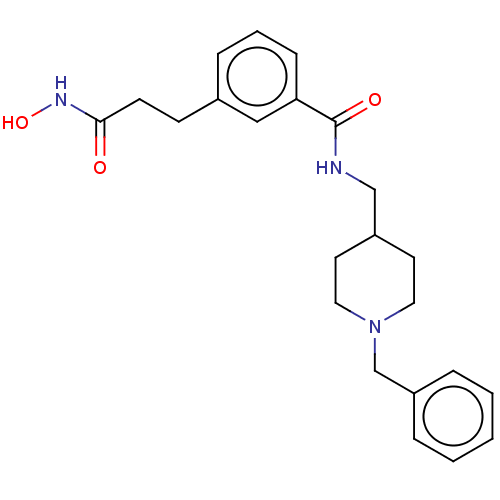
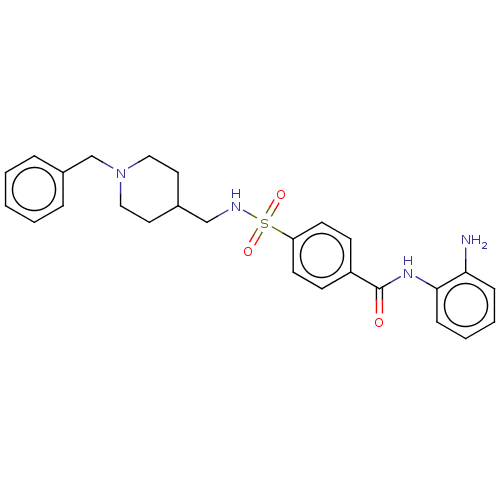
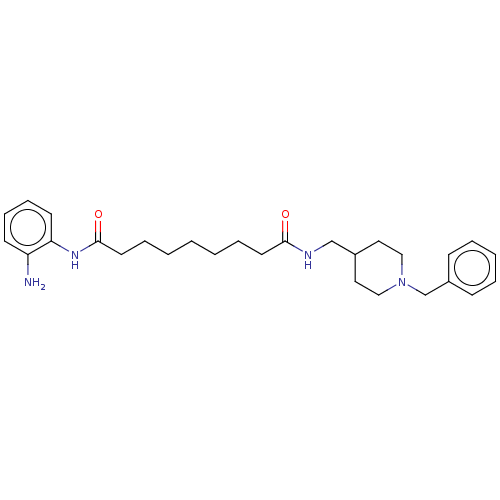
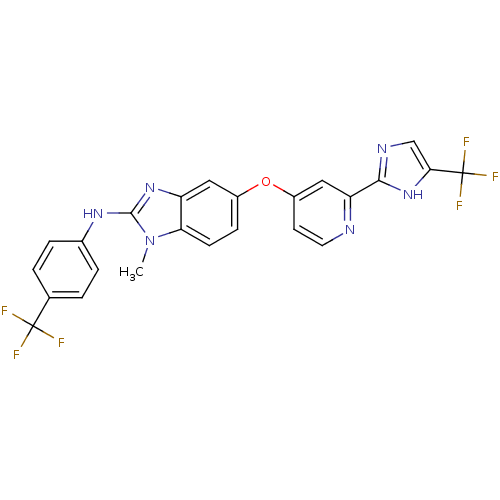
Affinity DataIC50: 4.20nMAssay Description:Inhibition of EGFR after 1 hrs by luminescence assayMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2Z89CSBPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2Z89CSBPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 20nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 20nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 40nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 50nMAssay Description:In vitro inhibitory activity against histamine H2-receptor in isolated Guinea pig right atria.More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 67nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 70nMAssay Description:The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2)More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 140nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 170nMAssay Description:The compound was tested in vitro for inhibition of rat hepatic HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 180nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 280nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 430nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 450nMAssay Description:The compound was tested in vitro for inhibition of rat hepatic HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 460nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 470nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 490nMAssay Description:The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 590nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 800nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.00E+3nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 1.14E+3nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Tsinghua University
Curated by ChEMBL
Tsinghua University
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of RAFMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.25E+3nMAssay Description:The compound was tested in vitro for inhibition of rat hepatic HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 1.28E+3nMAssay Description:The compound was tested in vitro for inhibition of rat hepatic HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Tsinghua University
Curated by ChEMBL
Tsinghua University
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 1.42E+3nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 1.62E+3nMAssay Description:In vitro for inhibition of rat HMG-CoA reductase.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.62E+3nMAssay Description:Inhibitory activity against rat liver dihydrofolate reductaseMore data for this Ligand-Target Pair
In DepthDetails
Target InfoPDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of EGFR after 1 hrs by luminescence assayMore data for this Ligand-Target Pair