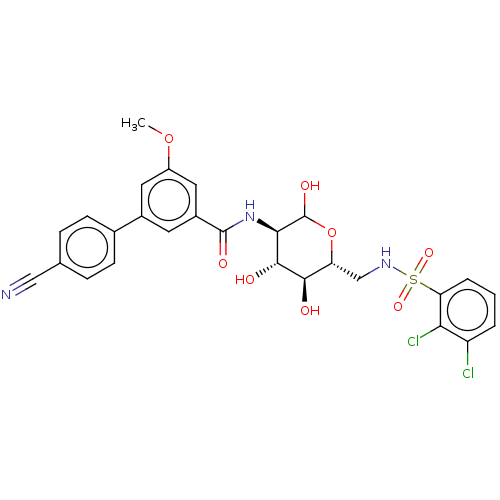
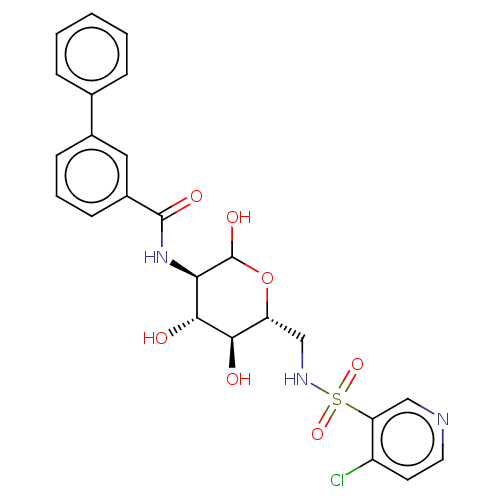
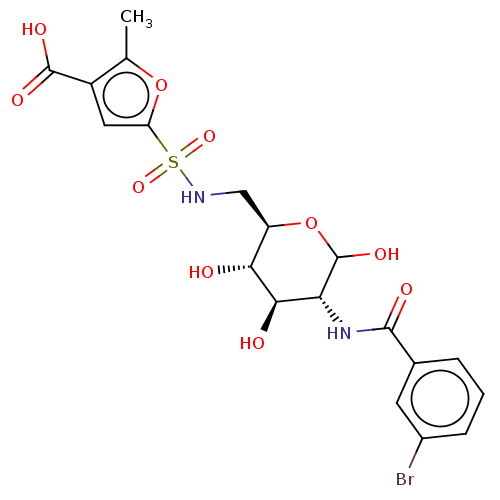
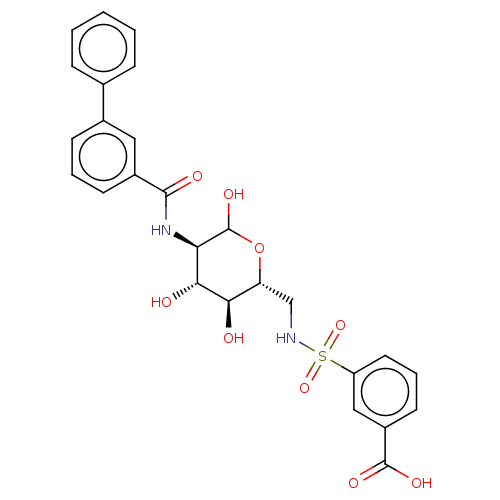
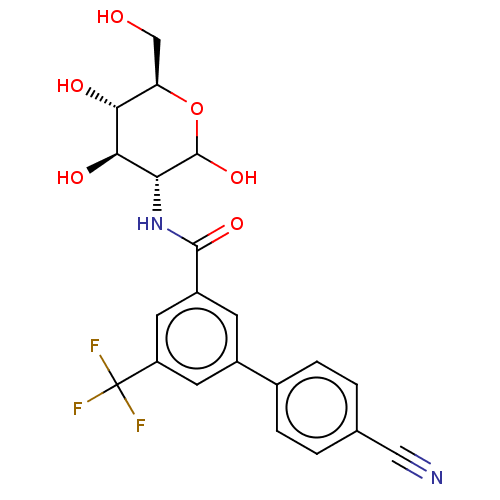
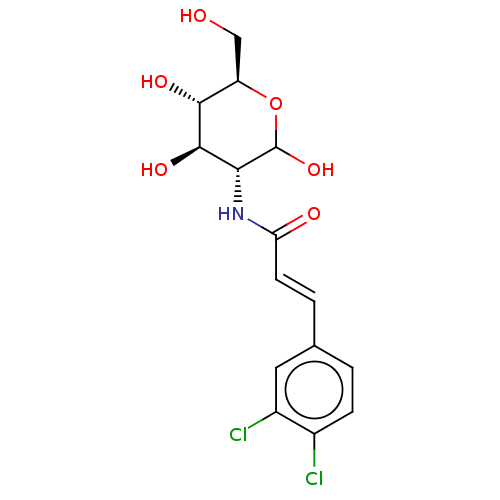
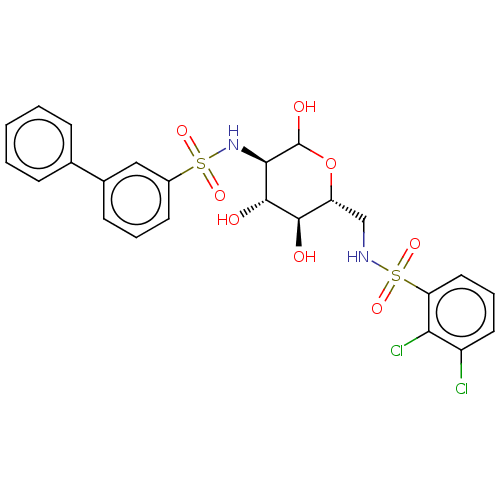
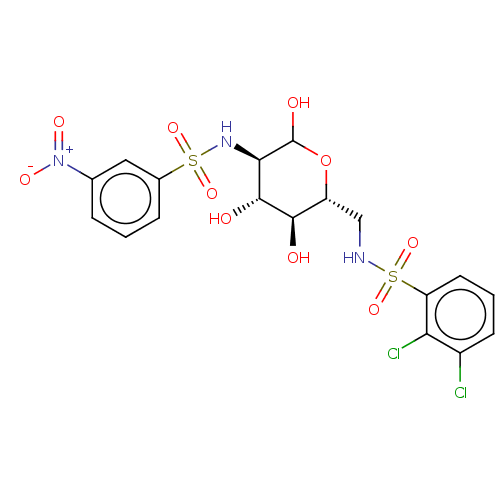
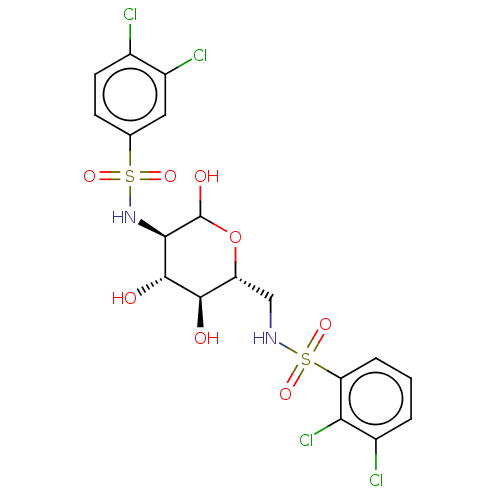
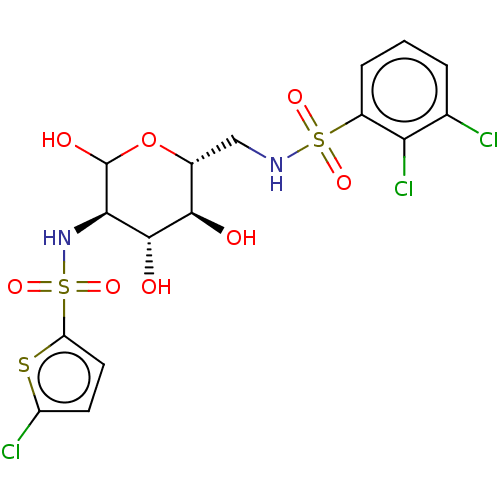
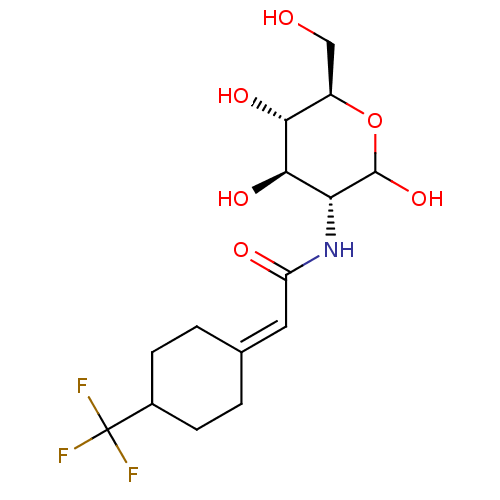
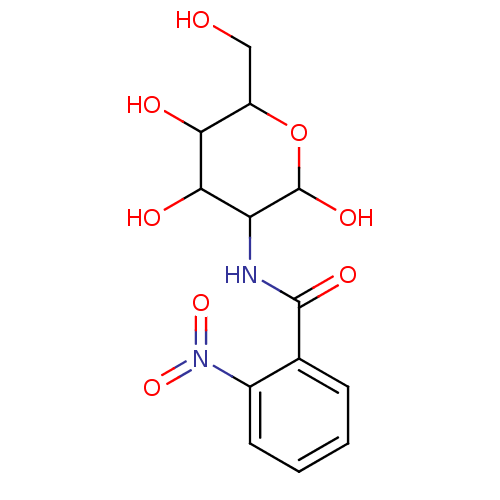
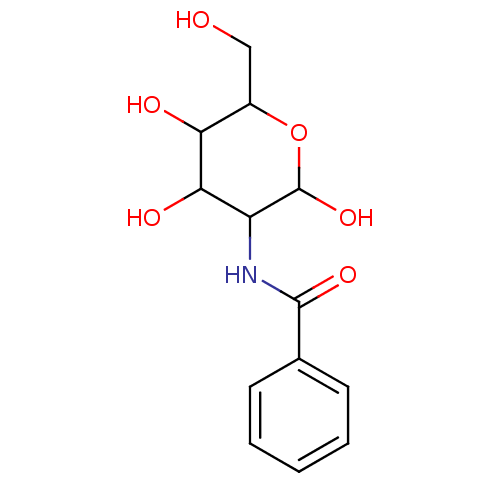
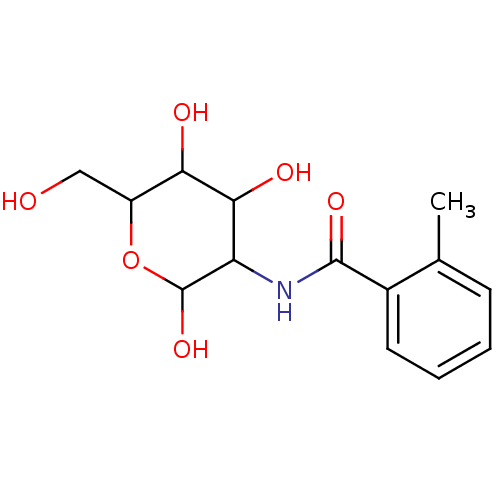
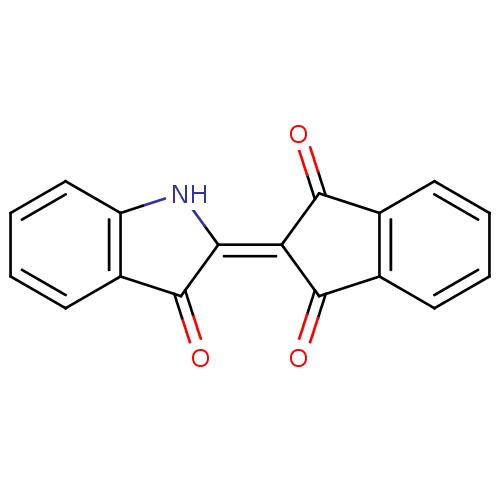
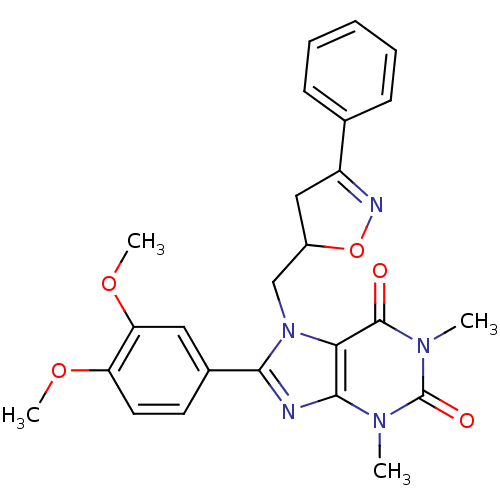
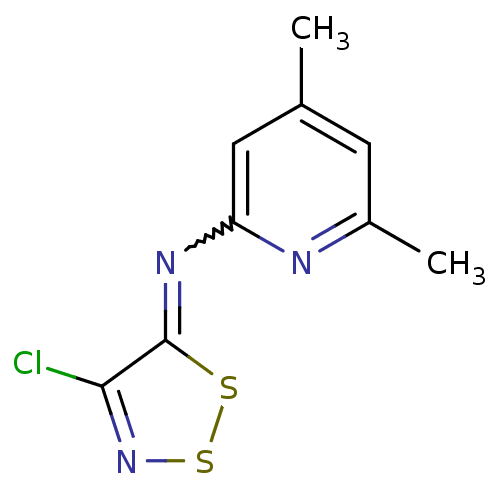
Affinity DataIC50: 7.90nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
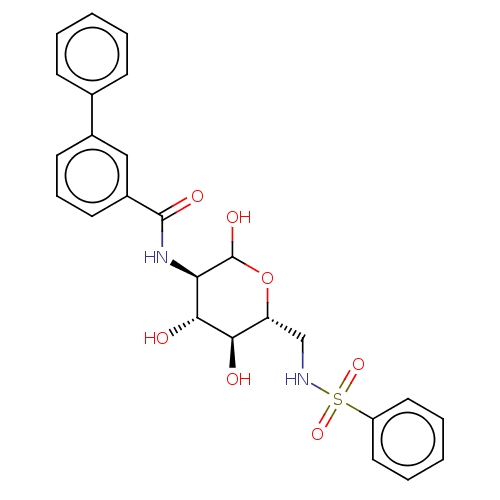
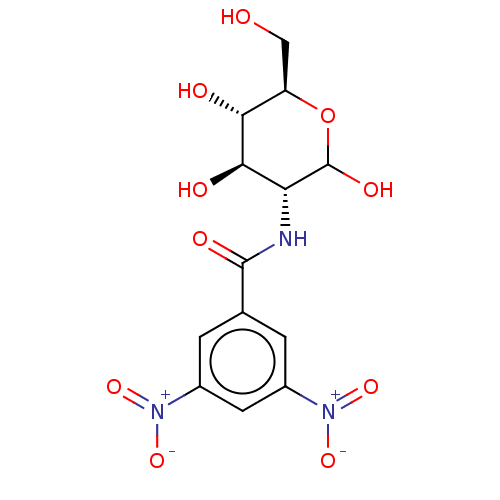
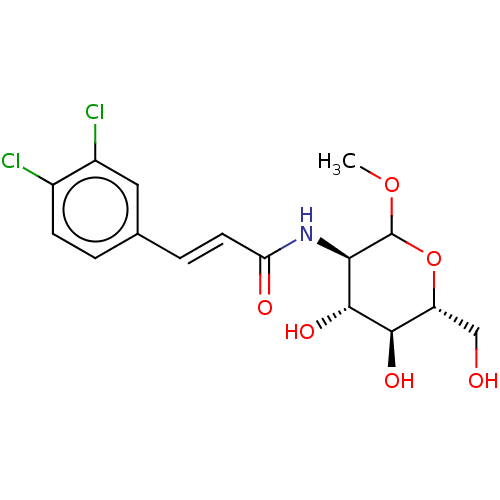
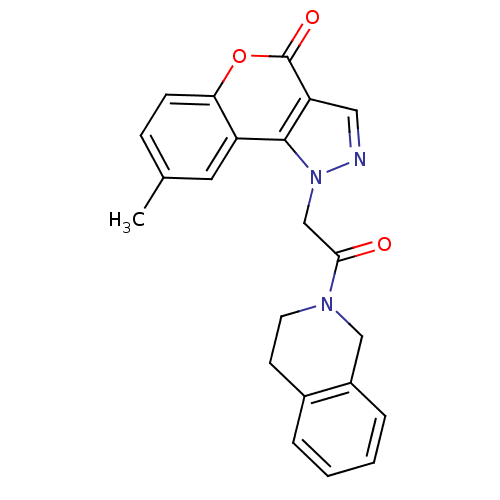
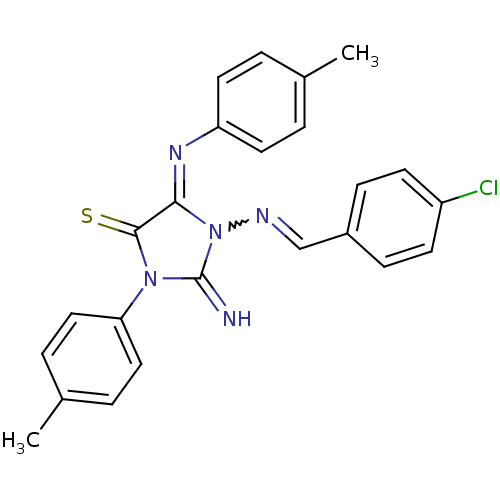
Affinity DataIC50: 16nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
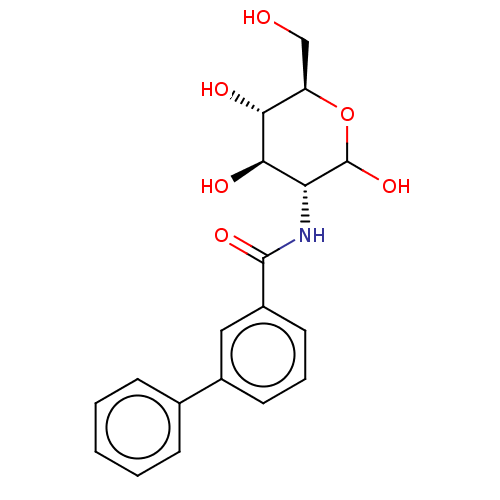
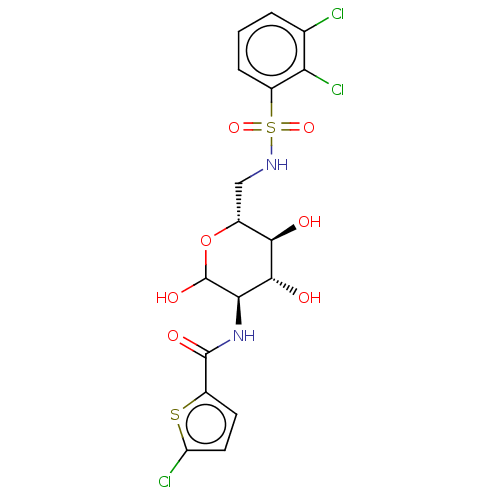
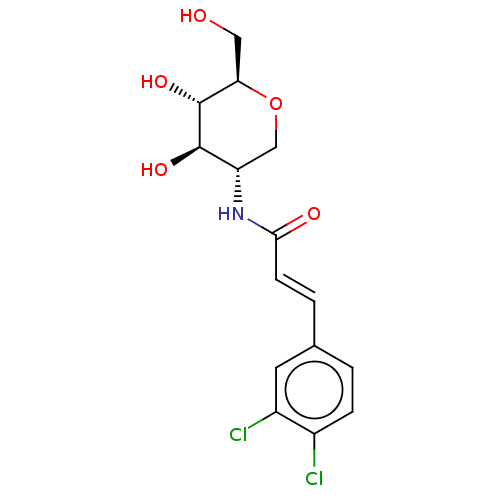
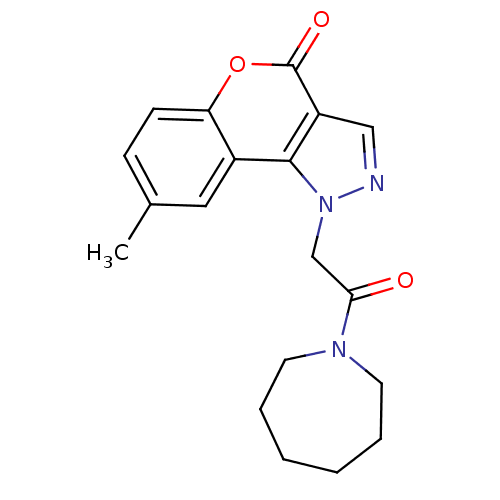
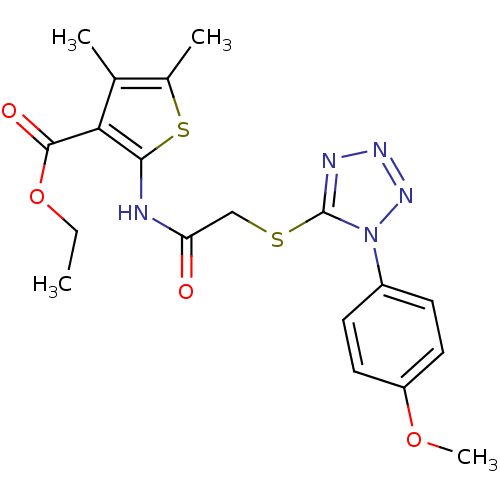
Affinity DataIC50: 20nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
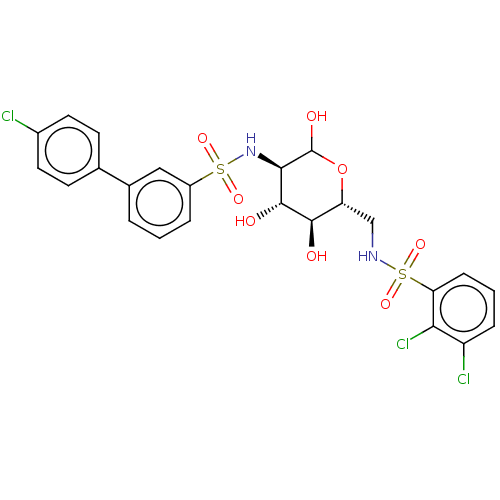
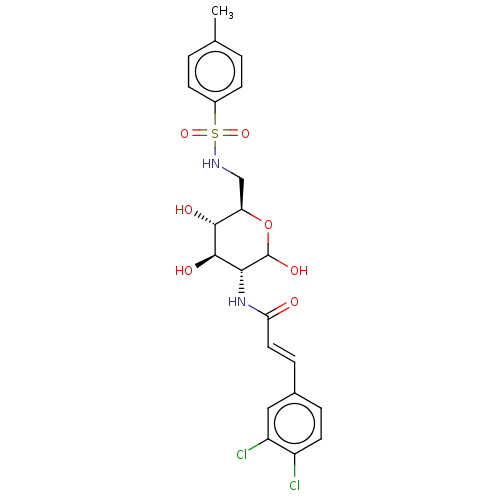
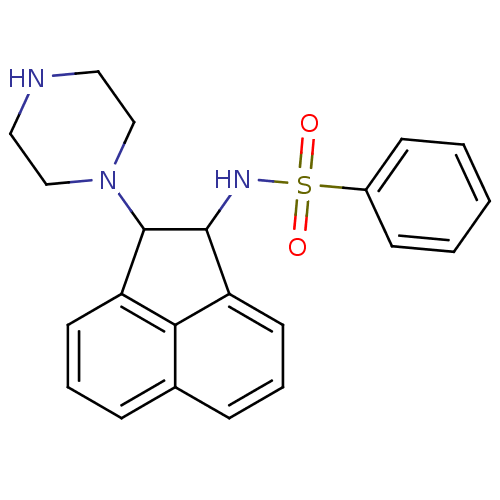
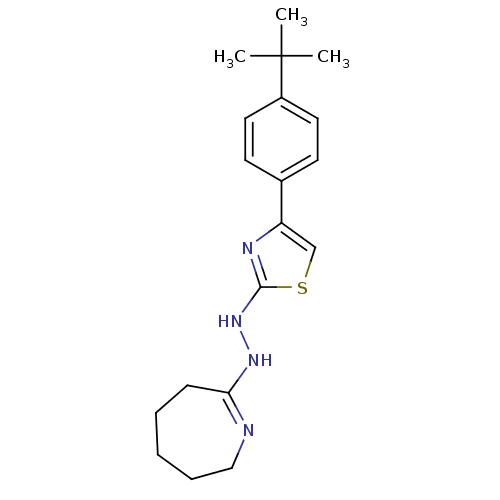
Affinity DataIC50: 20nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
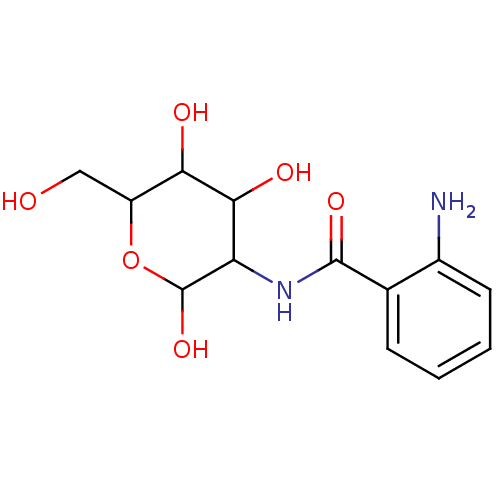
Affinity DataIC50: 25nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+4nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+5nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+6nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+7nMpH: 7.6 T: 2°CAssay Description:The assay phosphotransferase activity was followed spectrophotometrically b reduction of NADP in the presence of an excess of glucose-6-phosphate-deh...More data for this Ligand-Target Pair
Affinity DataEC50: 3.71E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 2.40E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 3.51E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 8.10E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: >8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 3.67E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 2.17E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 1.36E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 3.99E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: >8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 4.93E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 3.74E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 5.32E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 4.13E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 6.31E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 2.39E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 4.74E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: >8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair