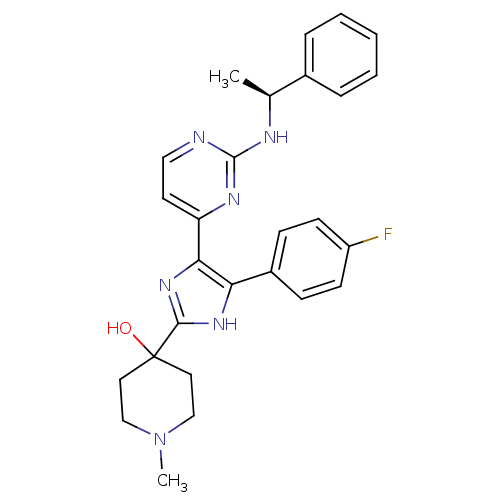
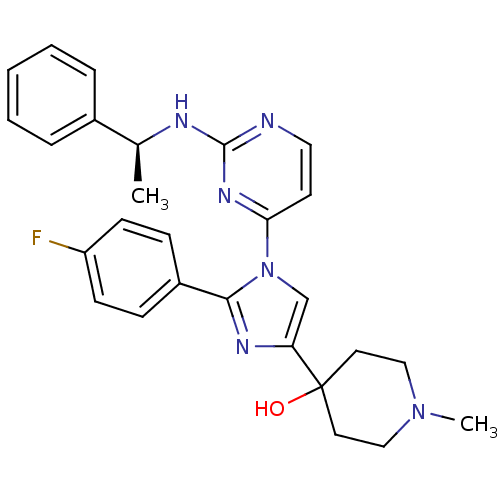
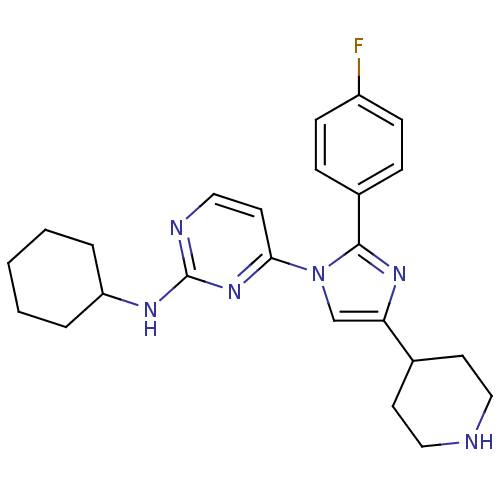
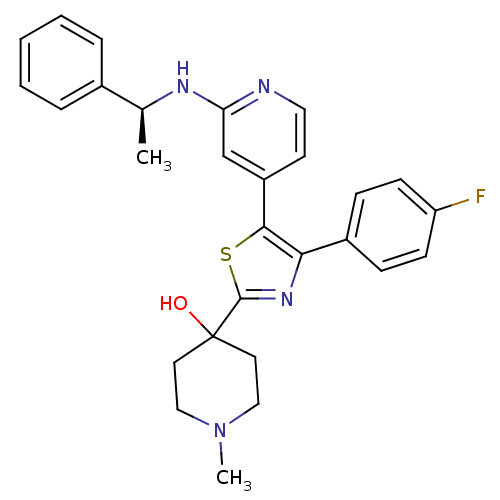
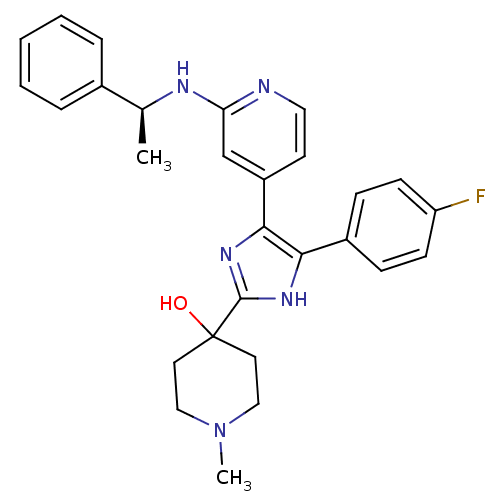
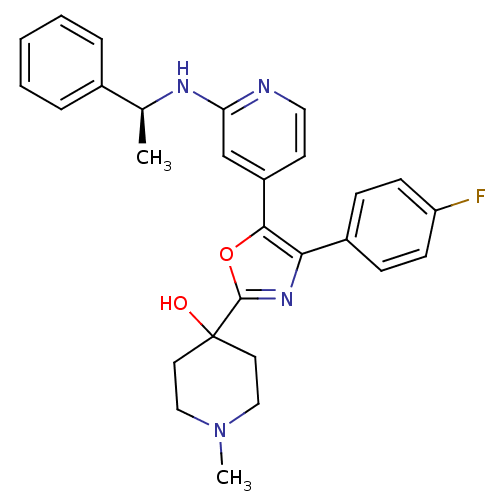
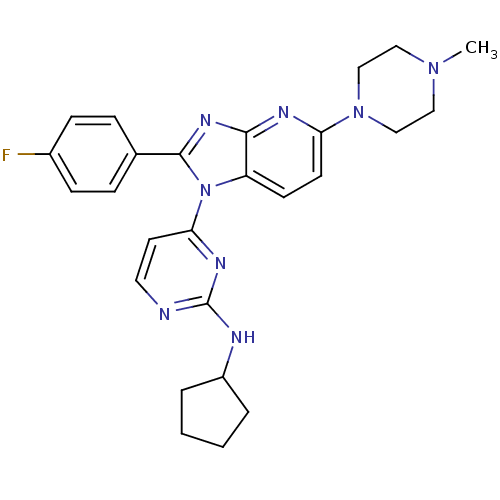
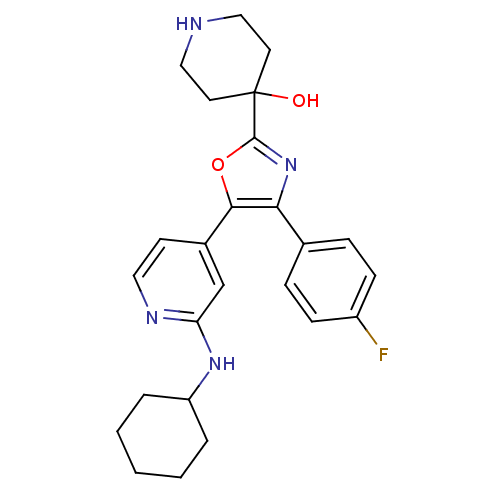
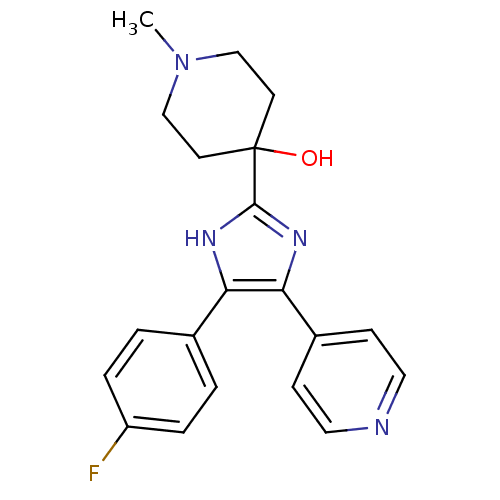
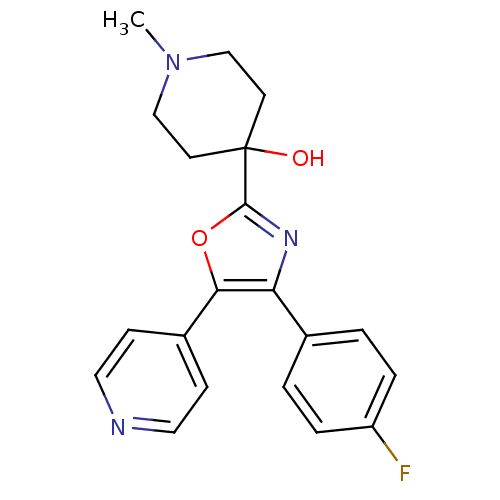
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human c-Jun N-terminal kinase 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of human c-Jun N-terminal kinase 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 8(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of human c-Jun N-terminal kinase 1More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 261nMAssay Description:Inhibition of human c-Jun N-terminal kinase 2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of human epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Mus musculus (mouse))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha.More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of human epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human Epidermal growth factor receptor, HER-1More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human vascular endothelial growth factor receptor 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 8(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human c-Jun N-terminal kinase 1More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human vascular endothelial growth factor receptor 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 8(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human c-Jun N-terminal kinase 1More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 1A2More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2C9More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 1A2More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2D6More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human Epidermal growth factor receptor, HER-1More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 2C9More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 1A2More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human cytochrome P450 1A2More data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human RAF proto-oncogene serine/threonine-protein kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 4.66E+3nMAssay Description:Inhibition of human c-Jun N-terminal kinase 2More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human c-SrcMore data for this Ligand-Target Pair