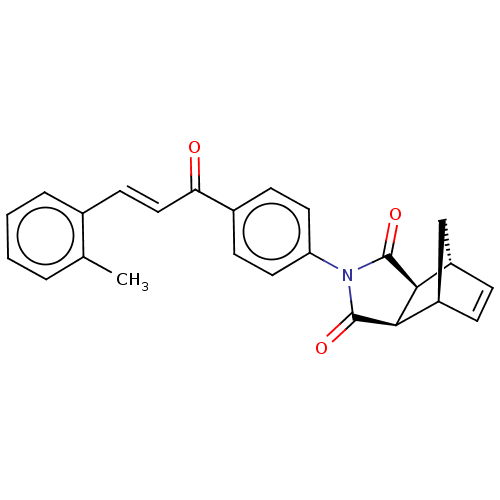
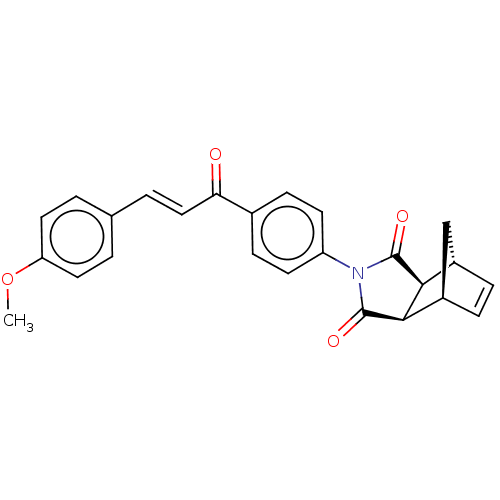
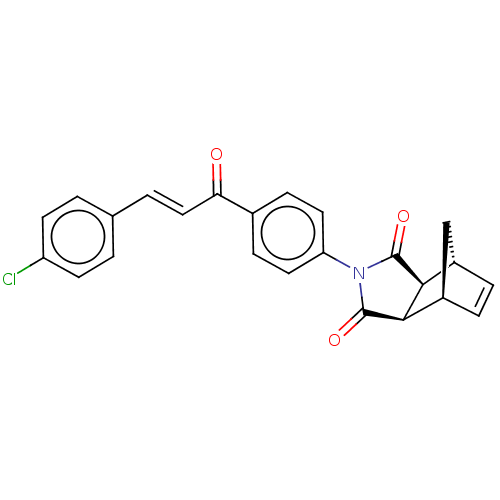
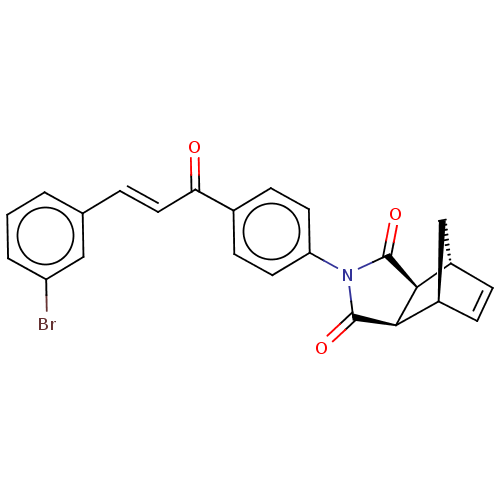
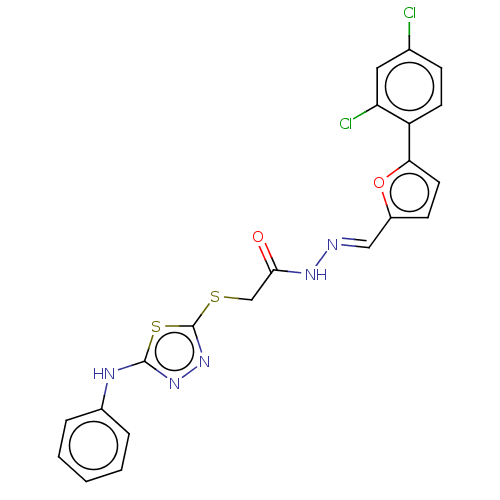
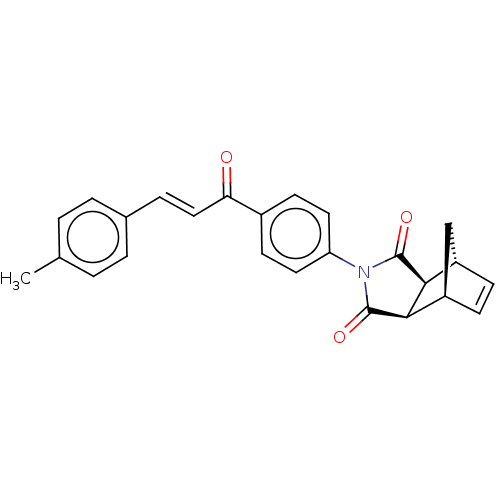
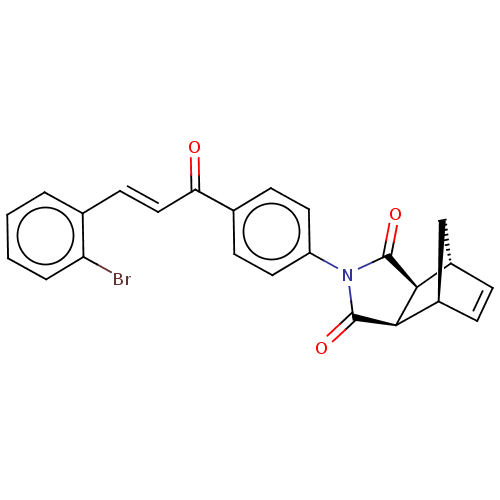
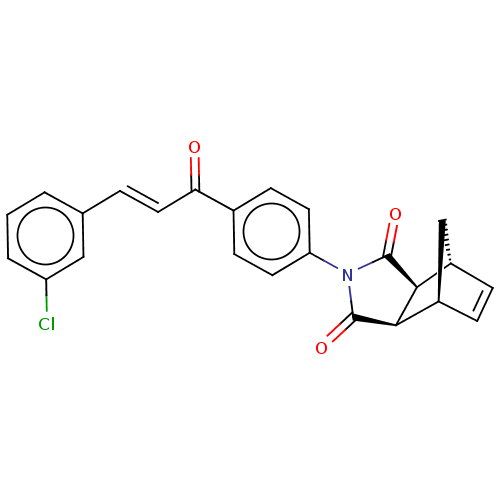
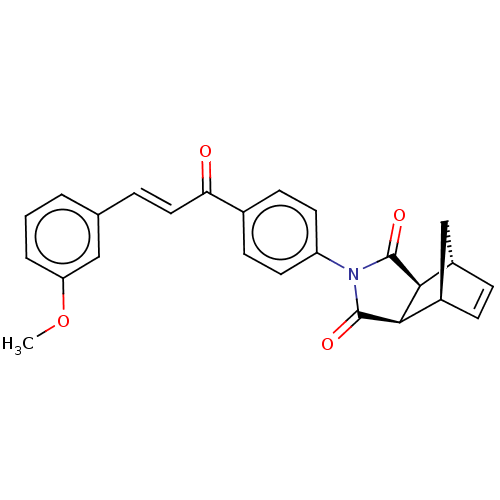
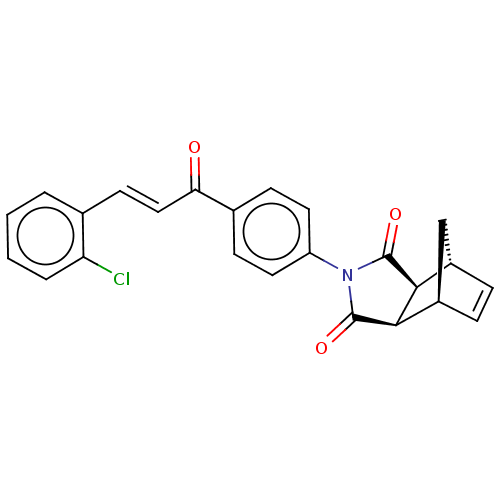
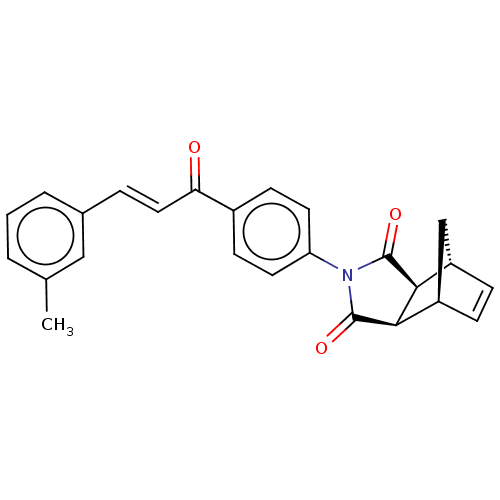
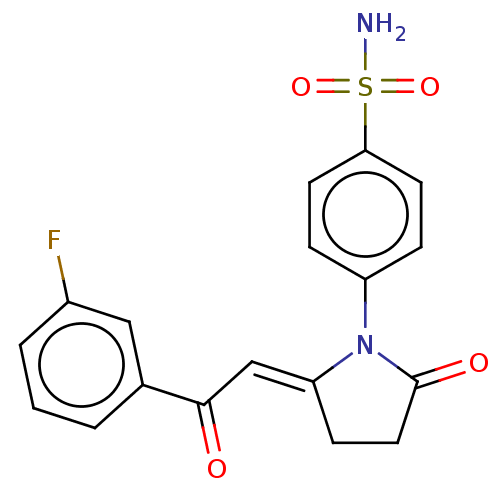
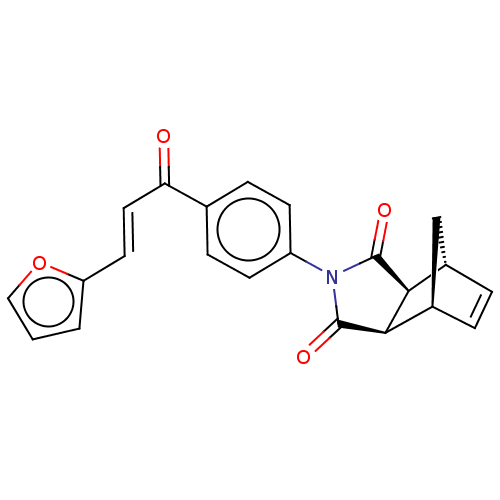
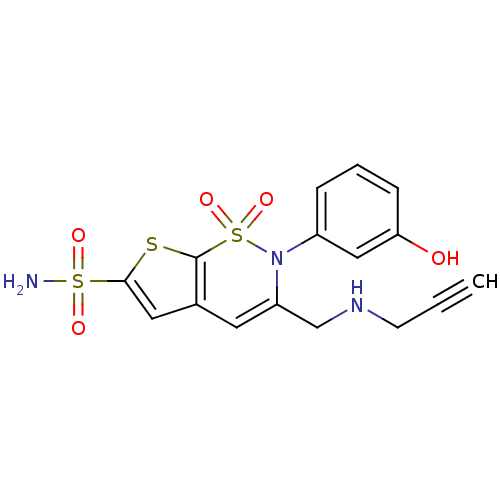
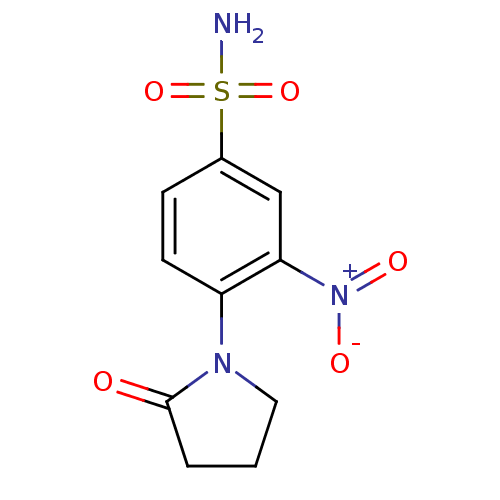
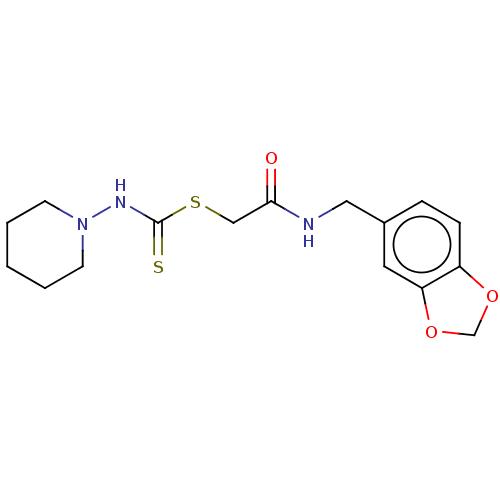
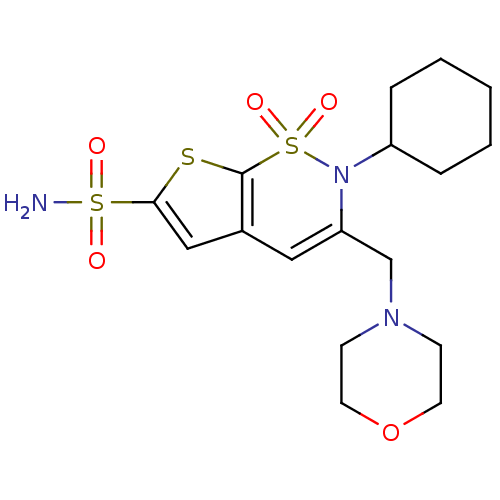
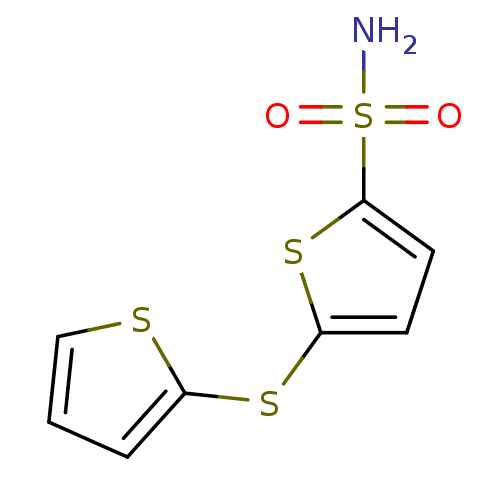
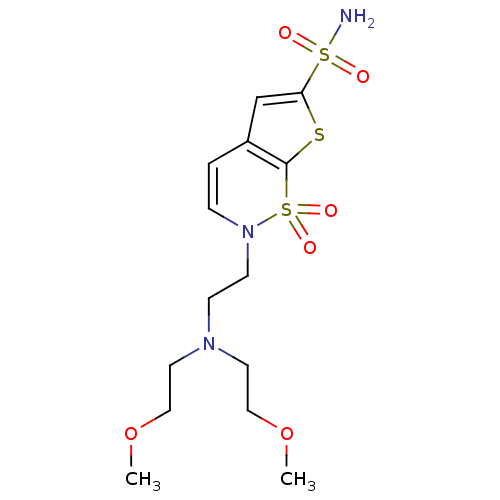
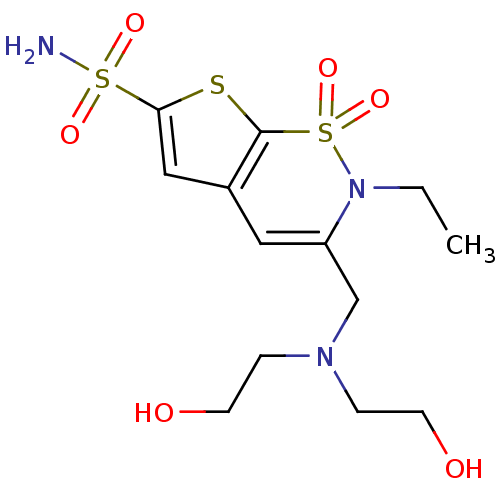
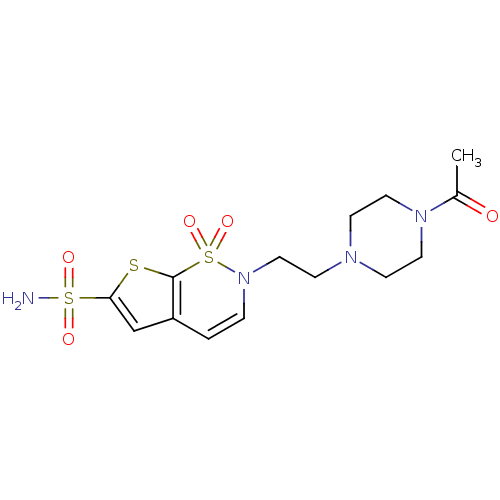
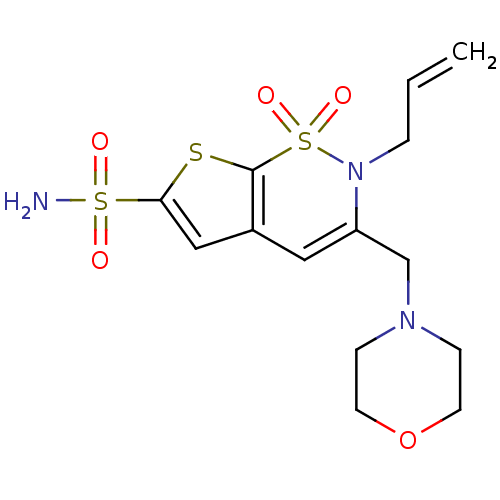
Affinity DataIC50: 0nMAssay Description:Inhibition of human CA2 by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMAssay Description:Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II)More data for this Ligand-Target Pair
Affinity DataIC50: 0.287nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of full length carbonic anhydrase-2 in human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.338nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataKi: 0.245nM ΔG°: -13.1kcal/mole IC50: 0.352nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.302nM ΔG°: -13.0kcal/mole IC50: 0.353nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.277nM ΔG°: -13.0kcal/mole IC50: 0.356nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.258nM ΔG°: -13.1kcal/mole IC50: 0.373nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.343nM ΔG°: -12.9kcal/mole IC50: 0.382nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Protein kinase C alpha expressed in Sf-9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nM ΔG°: -12.8kcal/mole IC50: 0.406nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataKi: 0.379nM ΔG°: -12.8kcal/mole IC50: 0.415nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.361nM ΔG°: -12.9kcal/mole IC50: 0.419nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.337nM ΔG°: -12.9kcal/mole IC50: 0.435nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.377nM ΔG°: -12.8kcal/mole IC50: 0.444nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.404nM ΔG°: -12.8kcal/mole IC50: 0.449nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of Protein kinase C eta expressed in Sf-9 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of Protein kinase C eta expressed in Sf-9 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataKi: 0.343nM ΔG°: -12.9kcal/mole IC50: 0.485nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2CF9NZBPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2CF9NZBPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataKi: 0.412nM ΔG°: -12.8kcal/mole IC50: 0.488nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II)More data for this Ligand-Target Pair
Affinity DataKi: 0.490nM ΔG°: -12.7kcal/mole IC50: 0.551nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataKi: 0.390nM ΔG°: -12.8kcal/mole IC50: 0.555nMpH: 7.4 T: 2°CAssay Description:Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMpH: 7.4 T: 2°CAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.622nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.651nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.790nMAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.799nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.802nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMpH: 7.4 T: 2°CAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMpH: 7.2 T: 2°CAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.855nMAssay Description:Inhibition of human erythrocyte carbonic anhydrase-2More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMpH: 7.4 T: 2°CAssay Description:Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMpH: 7.2 T: 2°CAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMpH: 7.2 T: 2°CAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:The assay measured the rate of CO2 hydration by determining the addition rate of a NaOH solution using a Radiometer VIT 90 titration system. The enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121...More data for this Ligand-Target Pair