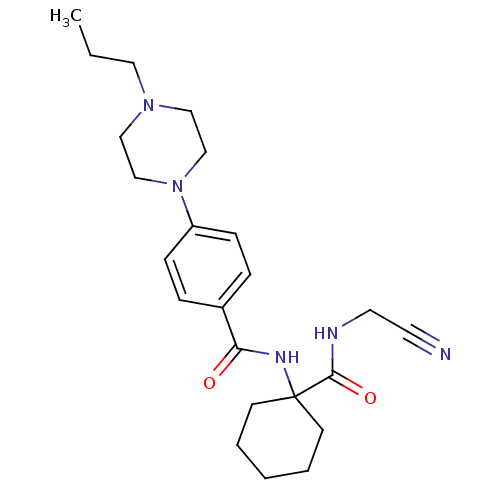
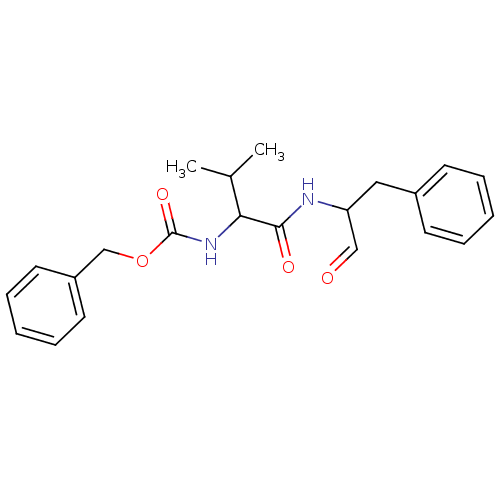
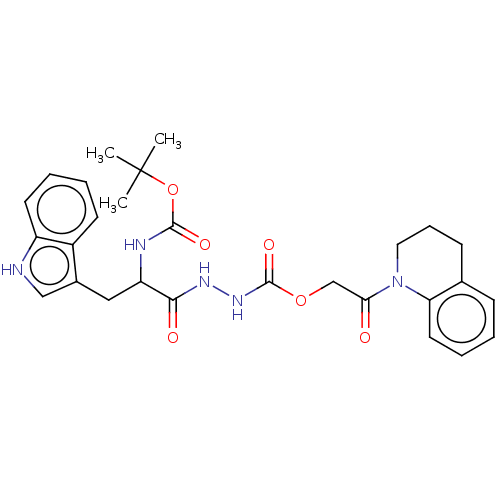
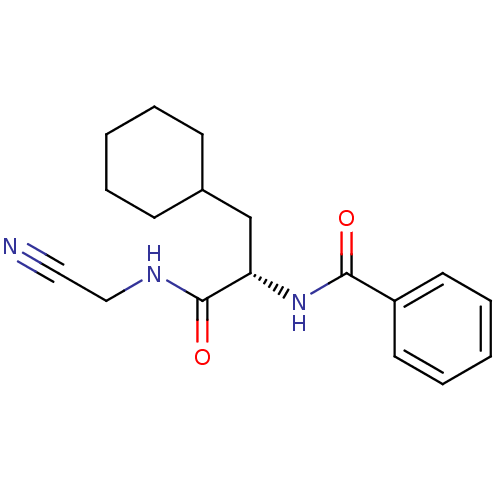
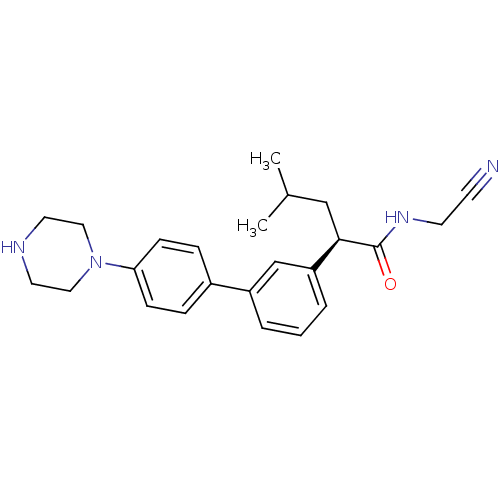
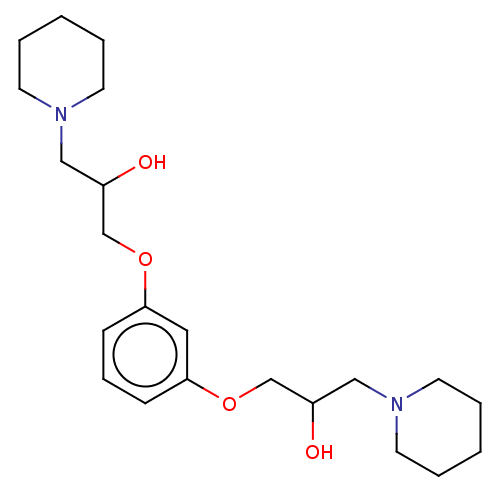
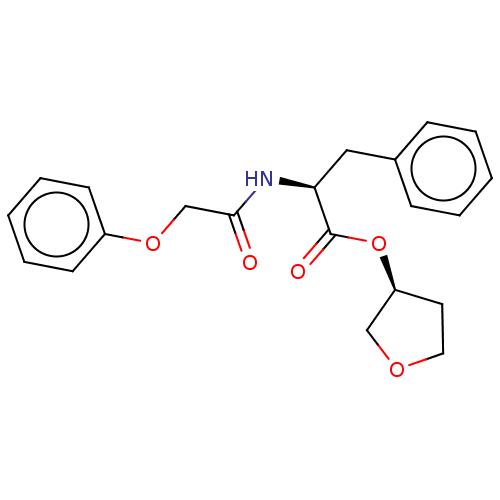
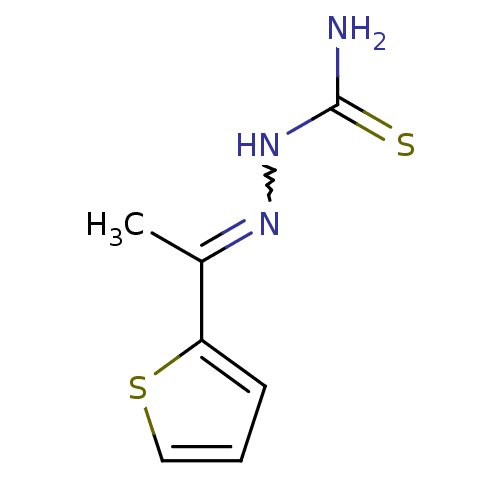
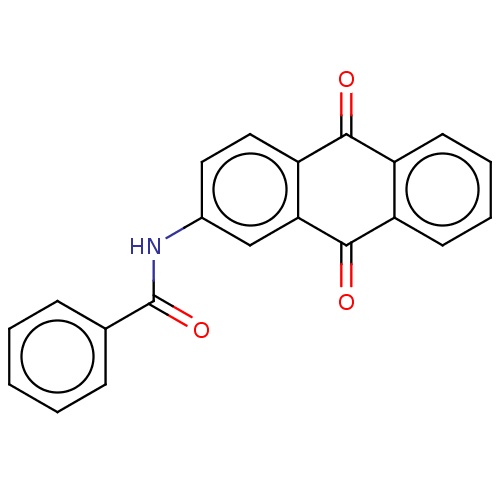
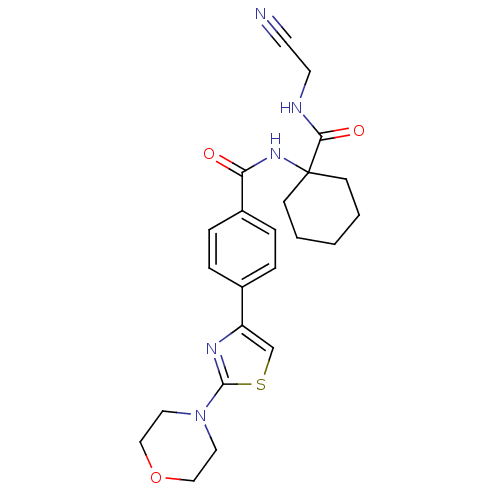
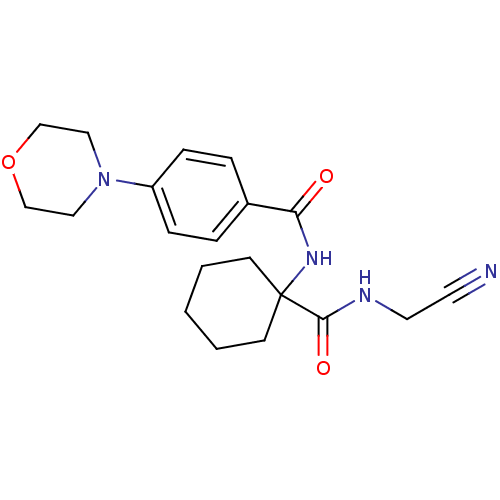
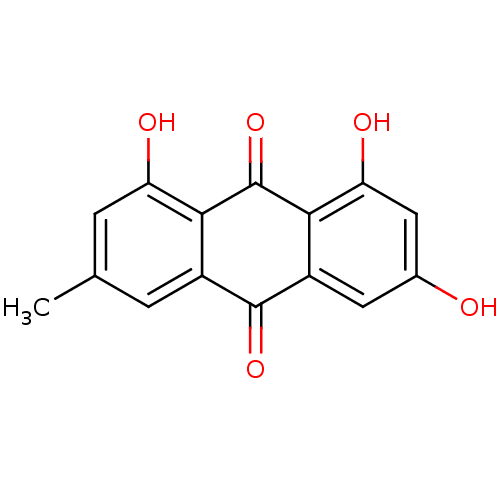
Affinity DataIC50: 503nM EC50: 48nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 193nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 55nM EC50: 230nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nM EC50: 320nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nM EC50: 340nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+3nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 6.00E+3nMAssay Description:Activation of human liver cathepsin L using Z-FR-AMC as substrate measured for 120 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6.40E+3nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 9.70E+3nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nM EC50: >1.00E+4nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nM EC50: >1.00E+4nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.00E+5nMAssay Description:This is a review article.More data for this Ligand-Target Pair