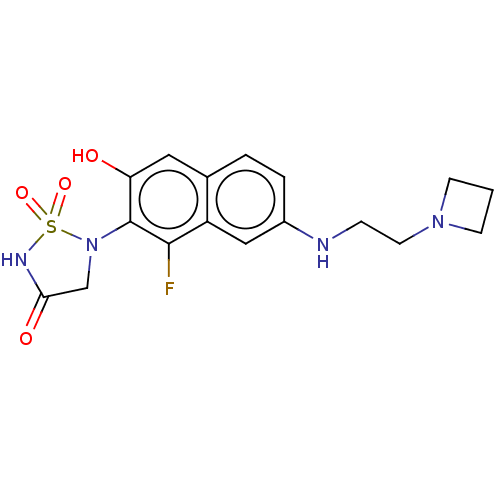
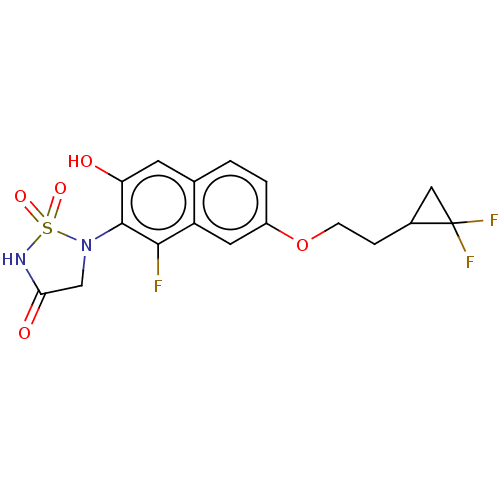
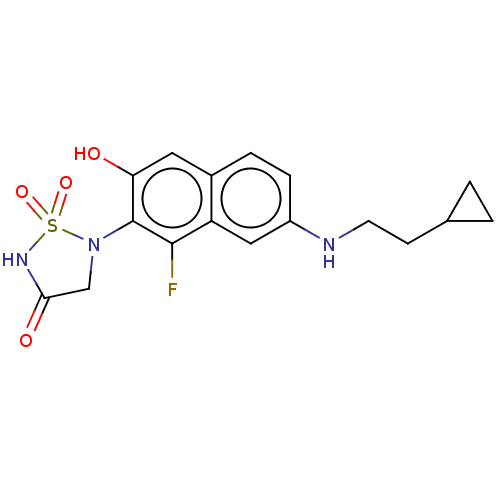
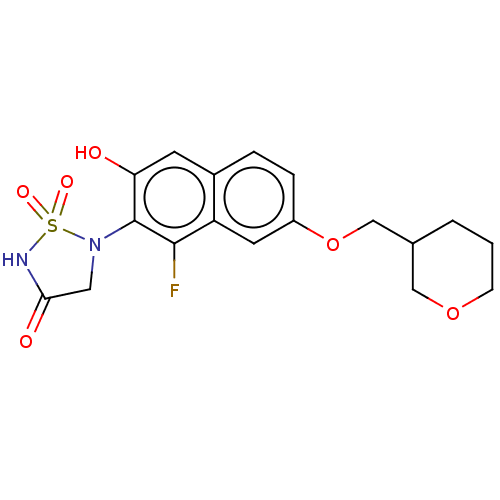
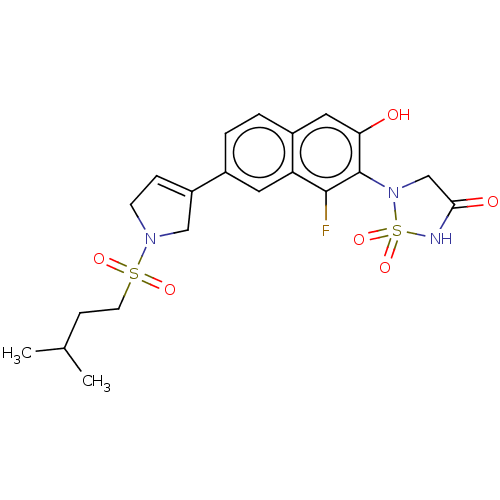
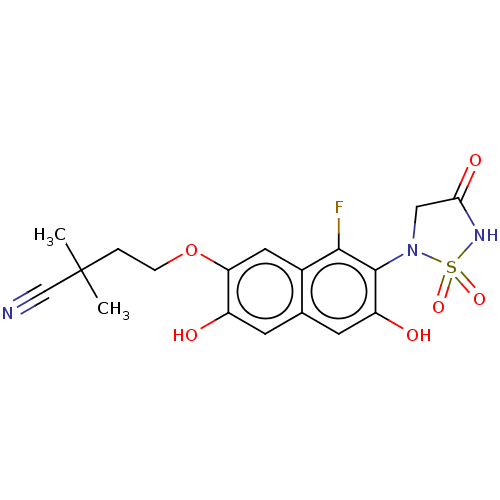
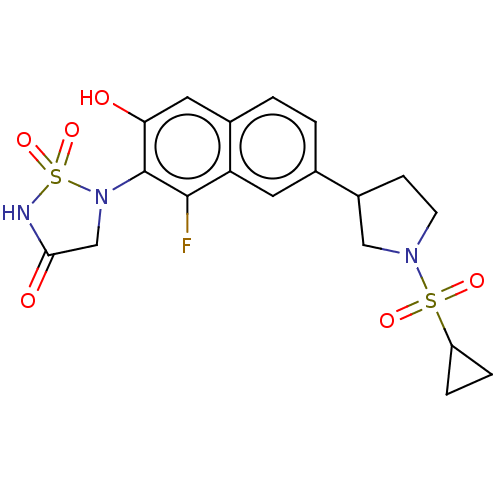
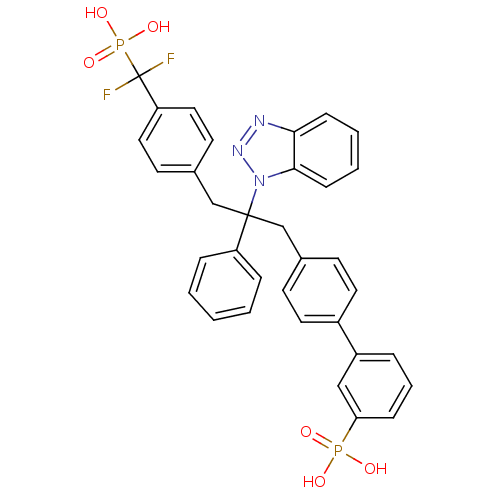
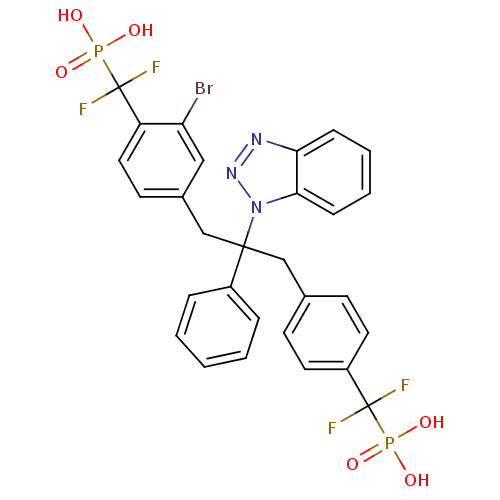
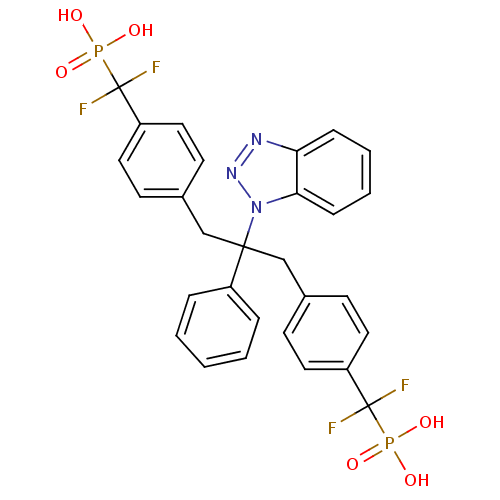
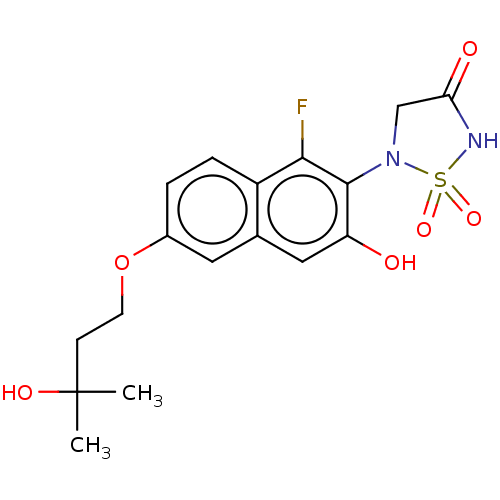
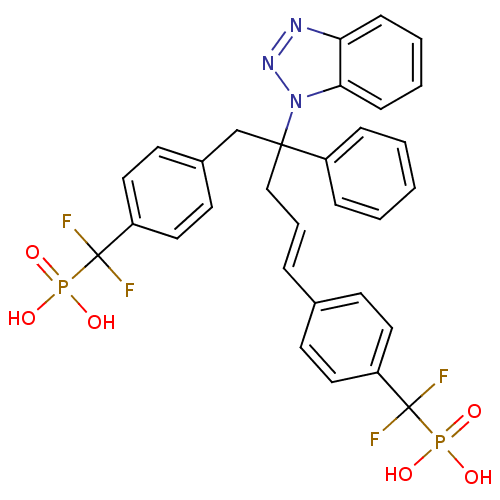
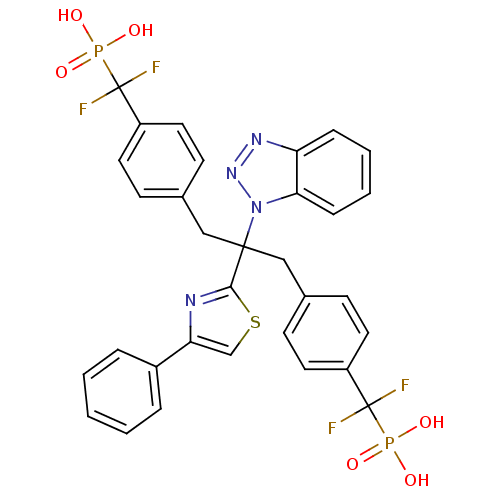
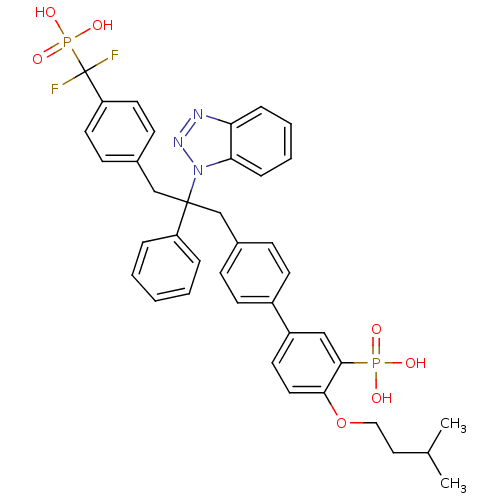
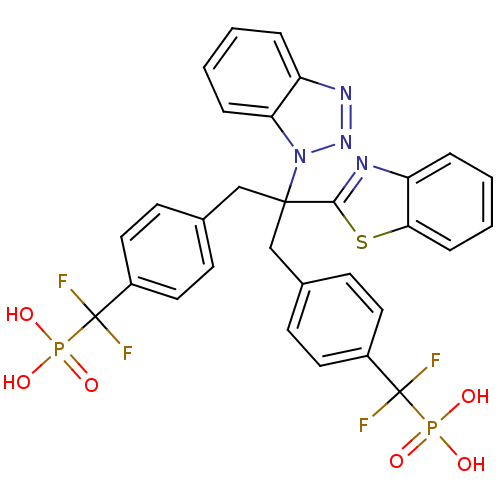
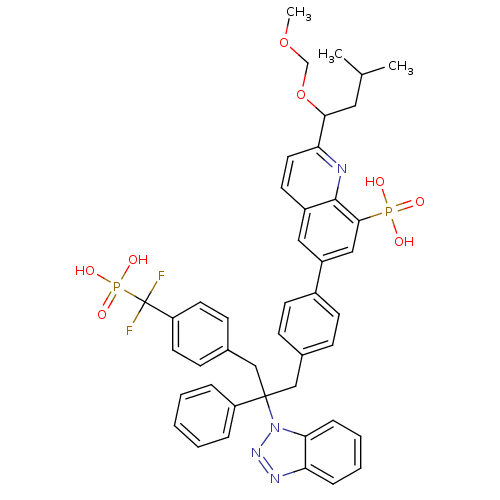
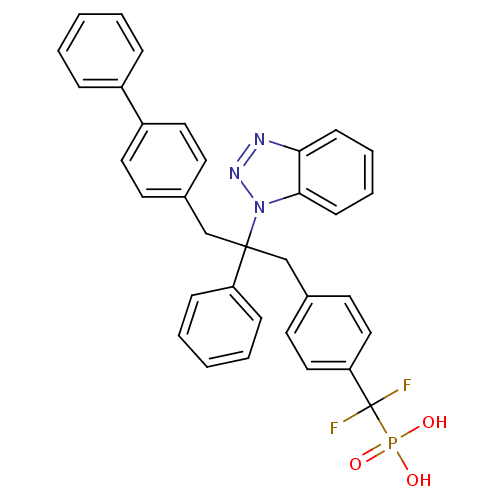
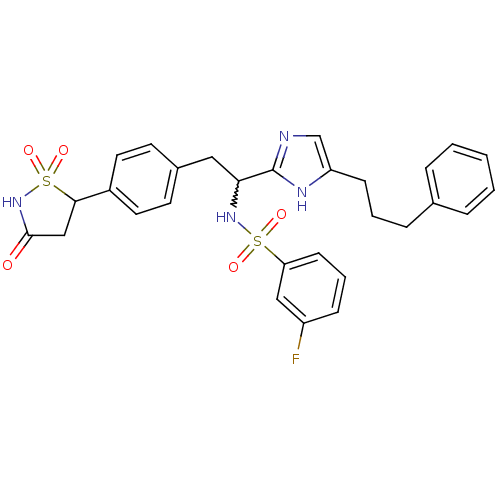
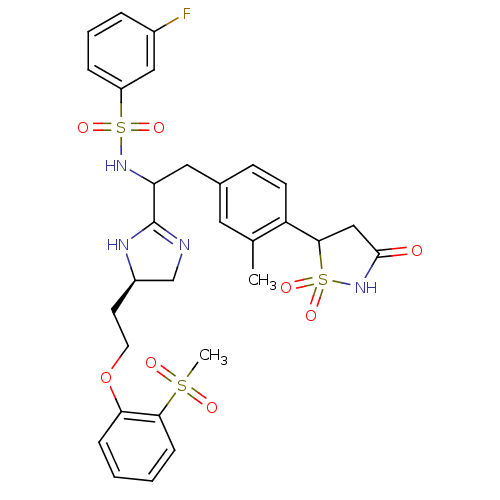
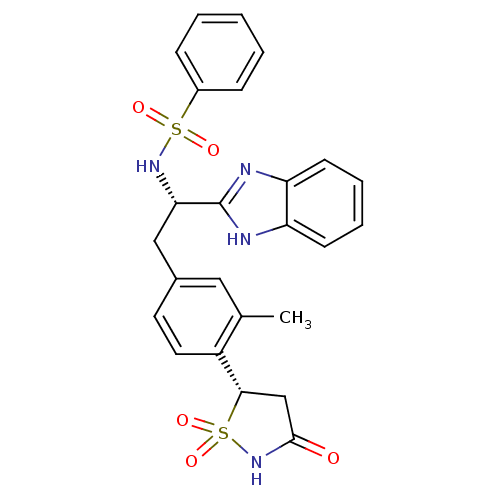
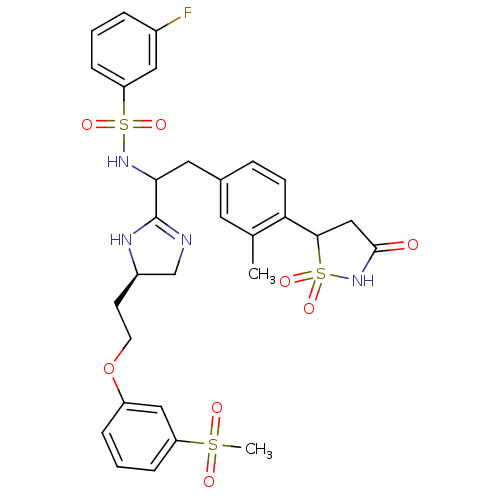
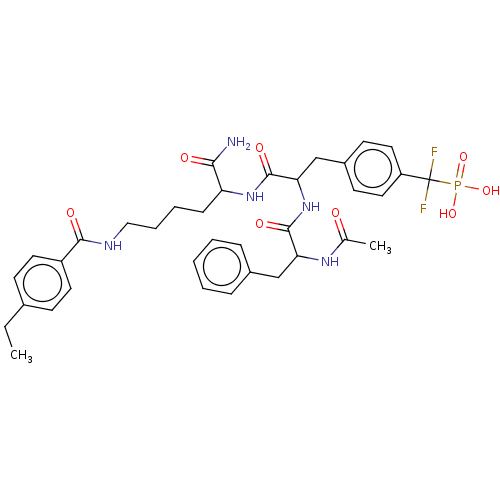
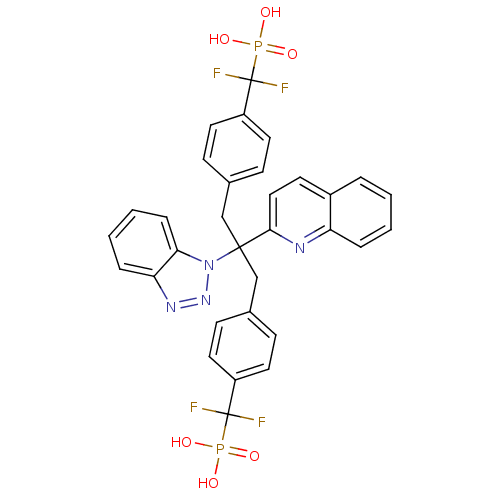
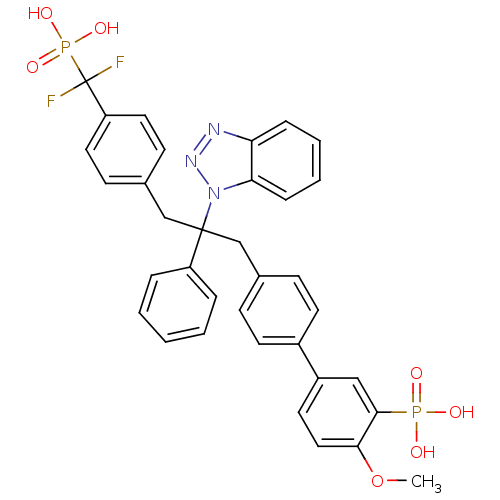
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of PTPN2 (unknown origin) by Mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant human TCPTP using pNPP as substrate measured after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant TCPTP assessed as inhibition of hydrolysis of p-nitrophenol after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant TCPTP (unknown origin) using pNPP as substrate measured after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of TCPTP (unknown origin) using pNPP as substrate after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of TCPTP by pNPP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2 [V121L](Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Merck Frosst Center for Therapeutic Research
Affinity DataIC50: 24nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of TCPTP by pNPP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMpH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibitory activity against T cell protein tyrosine phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human FLAG-tagged TCPTP (1 to 296) expressed in Escherichia coli using fluorescein diphosphate as substrate by fluorescence based metho...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMpH: 6.3 T: 2°CAssay Description:Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of TCPTP by pNPP assayMore data for this Ligand-Target Pair