

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557772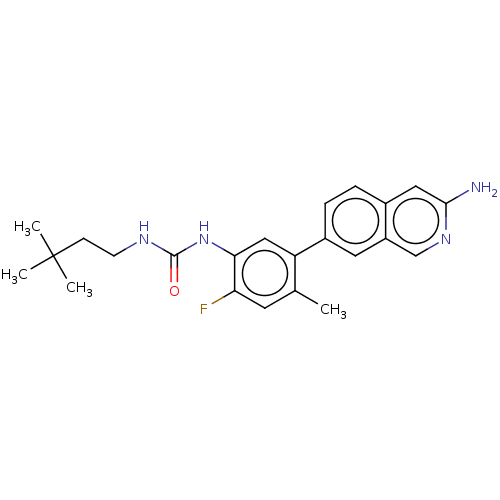 (CHEMBL4775998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580084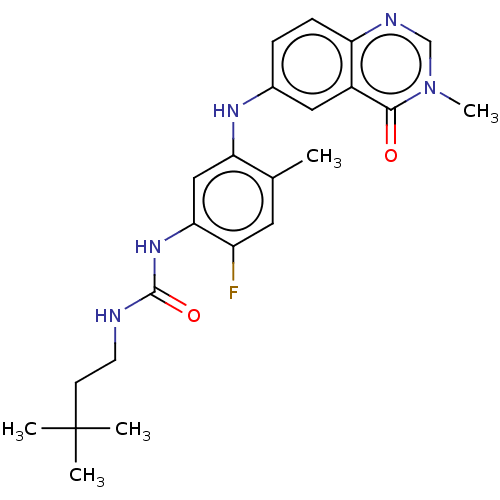 (CHEMBL5075174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580083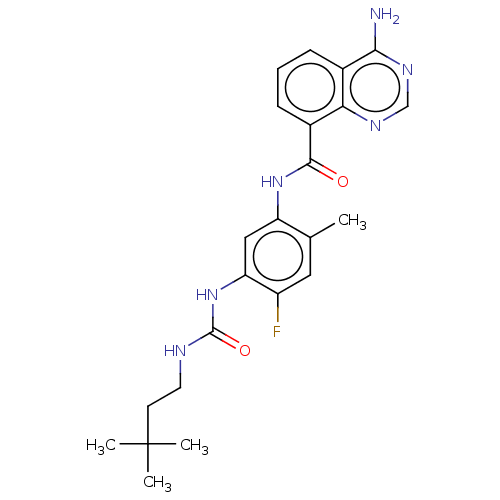 (CHEMBL5094268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580082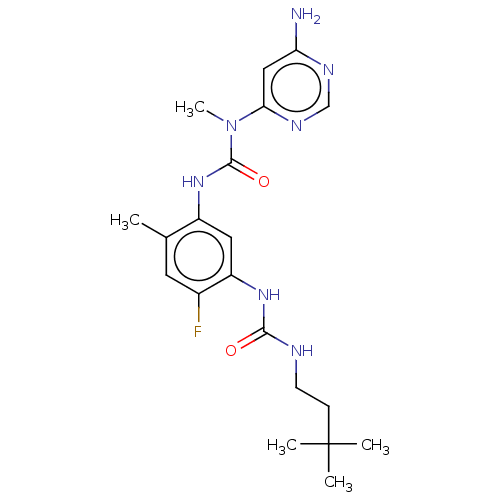 (CHEMBL5079215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580080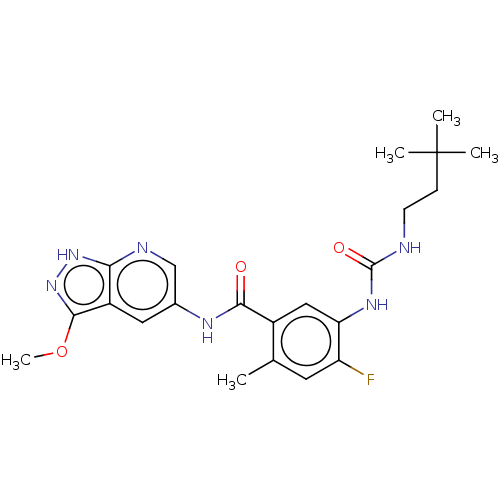 (CHEMBL5090624) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557775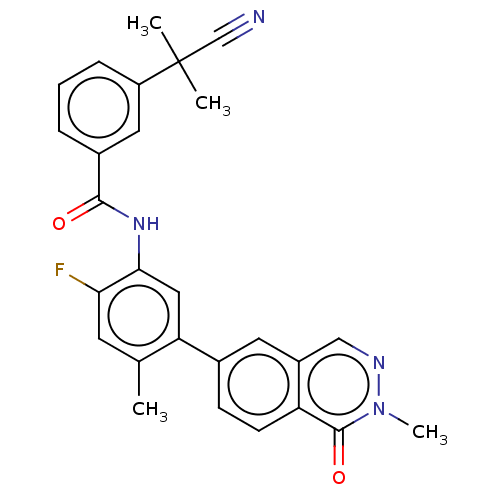 (CHEMBL4758903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557773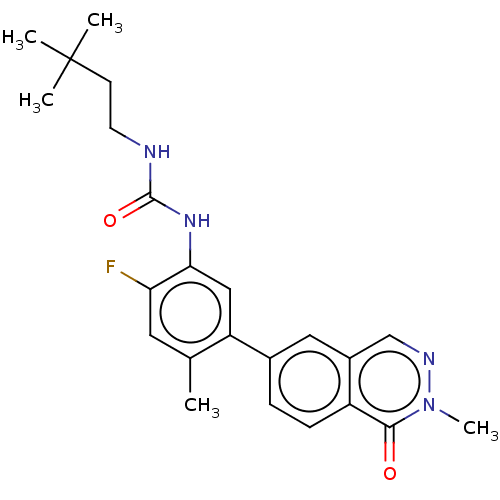 (CHEMBL4778772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50580081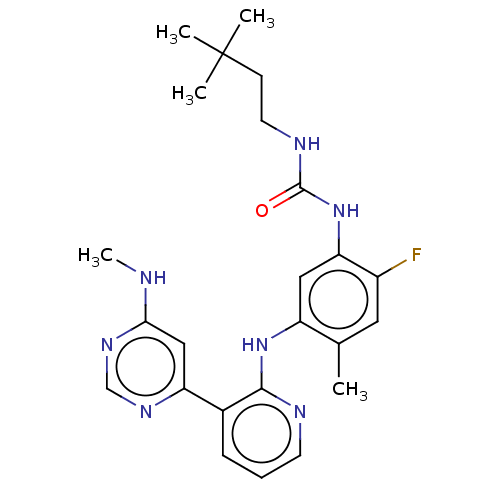 (CHEMBL5094514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557774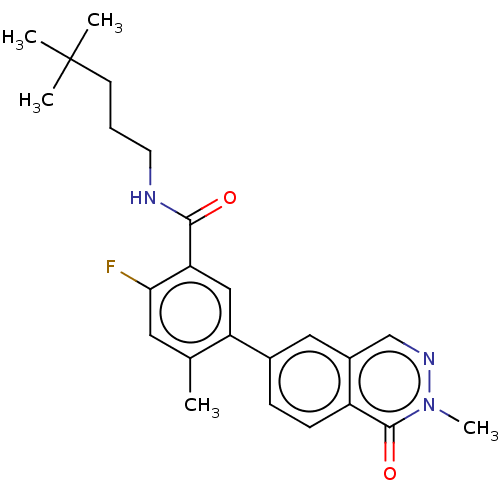 (CHEMBL4776565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50096279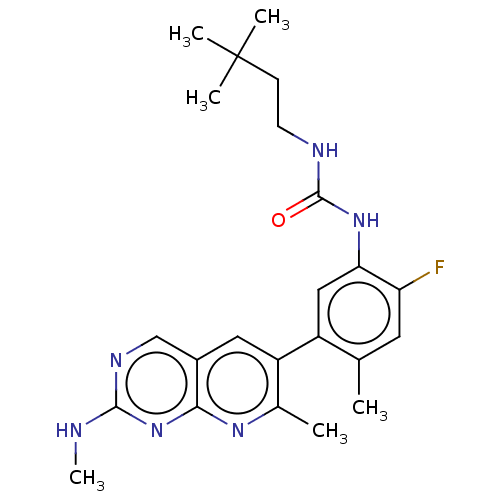 (CHEMBL3577124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50096279 (CHEMBL3577124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557770 (CHEMBL4780060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02085 BindingDB Entry DOI: 10.7270/Q2GT5S13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557776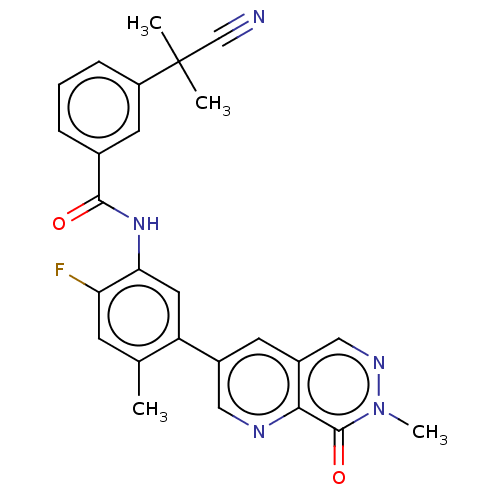 (CHEMBL4778419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50557771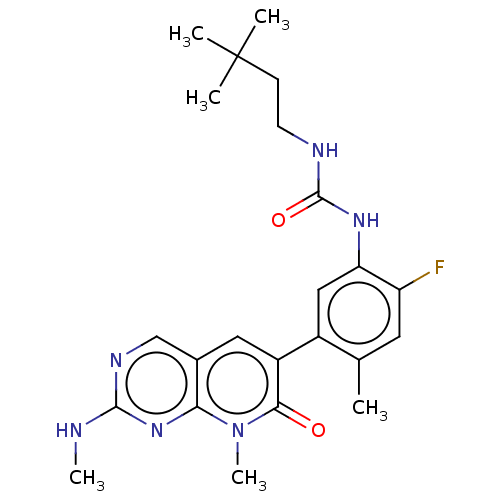 (CHEMBL4740241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00063 BindingDB Entry DOI: 10.7270/Q2DZ0D0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM35327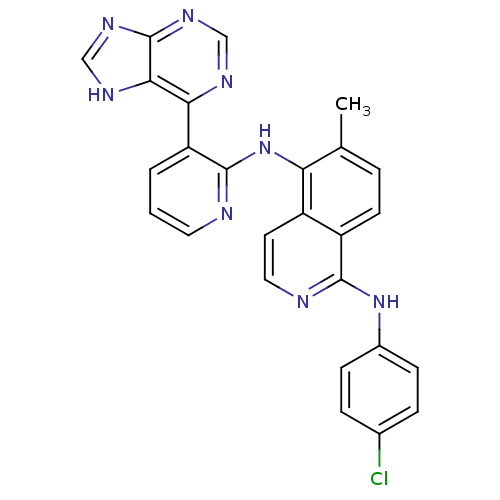 (pyridylpurine aminoisoquinoline, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 55: 7332-41 (2012) Article DOI: 10.1021/jm300613w BindingDB Entry DOI: 10.7270/Q29K4CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25628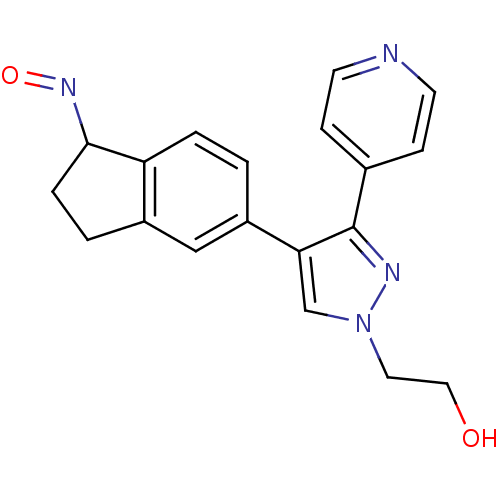 (2-{4-[(1Z)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 55: 7332-41 (2012) Article DOI: 10.1021/jm300613w BindingDB Entry DOI: 10.7270/Q29K4CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25391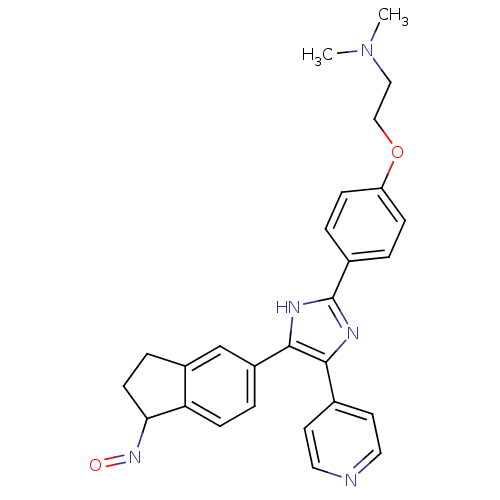 (CHEMBL200622 | SB-590885 | SB590885 | [2-(4-{4-[(1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 55: 7332-41 (2012) Article DOI: 10.1021/jm300613w BindingDB Entry DOI: 10.7270/Q29K4CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25617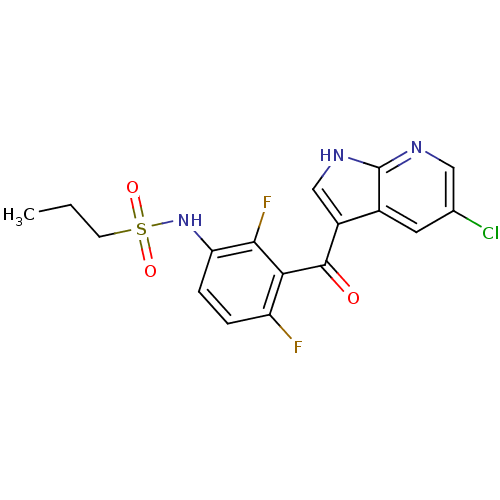 (N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 55: 7332-41 (2012) Article DOI: 10.1021/jm300613w BindingDB Entry DOI: 10.7270/Q29K4CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50427363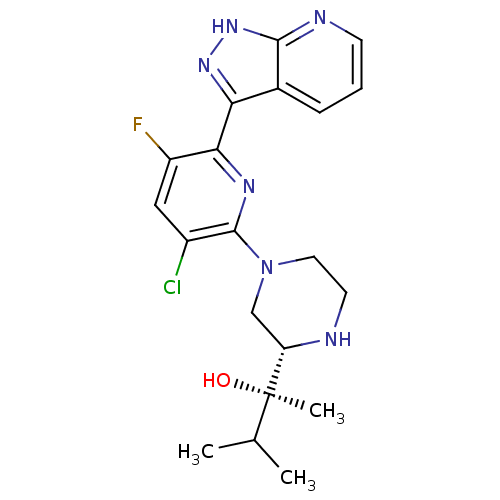 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Raf (unknown origin) | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50341519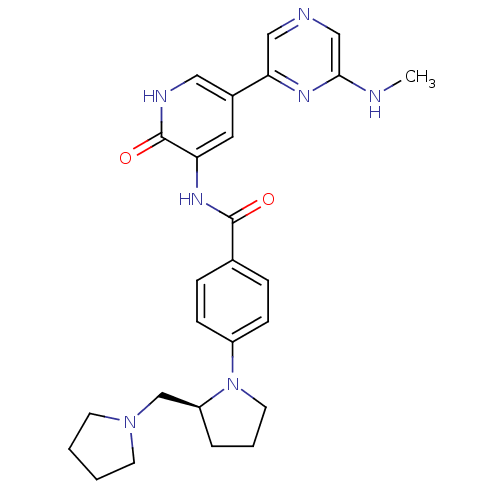 ((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of C-RAF | J Med Chem 54: 2341-50 (2011) Article DOI: 10.1021/jm101499u BindingDB Entry DOI: 10.7270/Q2KH0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of RAFc (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50463484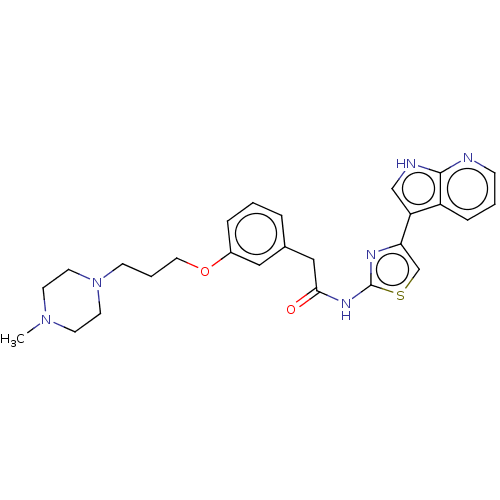 (CHEMBL4248525) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of RAFc (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410256 ((rac)-N-(3-(1,2,4,4a,5,6-hexahydro-[1,4]oxazino[4,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410350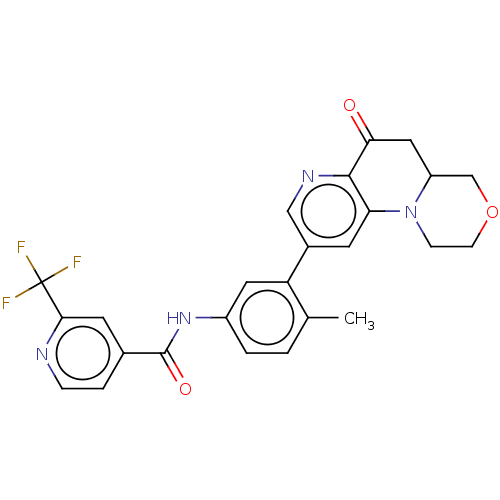 (US10377770, Example 110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410342 (N-(3-((4aR,6R)-6-Hydroxy-1,2,4,4a,5,6-hexahydro-[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410277 ((rac)-N-(3-(6-hydroxy-6-methyl-1,2,4,4a,5,6-hexahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410384 (US10377770, Example 144) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410356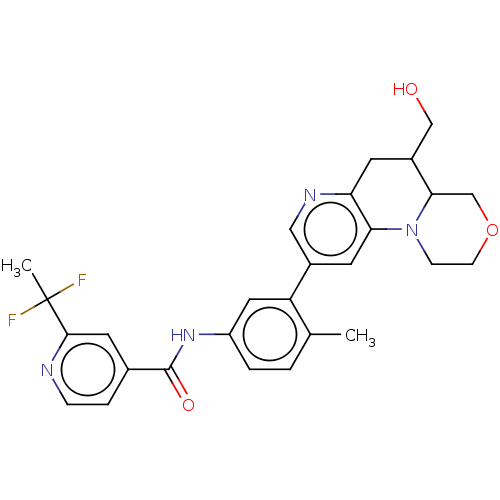 (US10377770, Example 116 | rac-trans-2-(1,1-Difluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410352 (US10377770, Example 112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410387 (US10377770, Example 147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410381 (US10377770, Example 141) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410363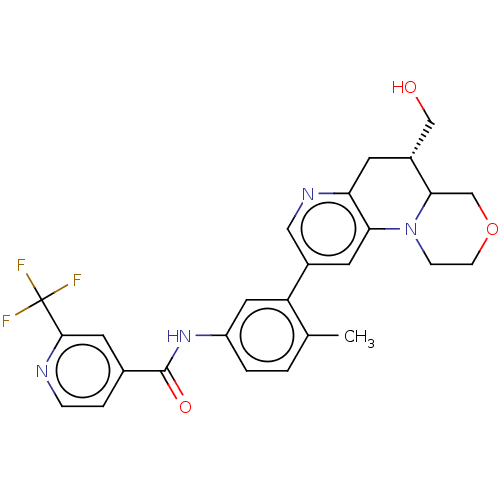 (US10377770, Example 123 | rac-trans-N-(3-(5-(Hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410362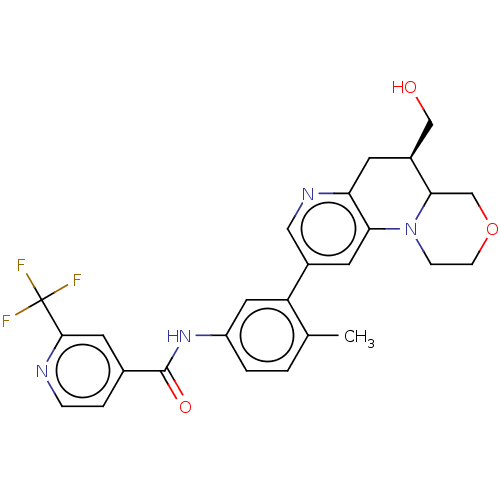 (US10377770, Example 122 | rac-trans-N-(3-(5-(Hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410357 (2-(1,1-Difluoroethyl)-N-(3-((4aR,5R)-5-(hydroxymet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410351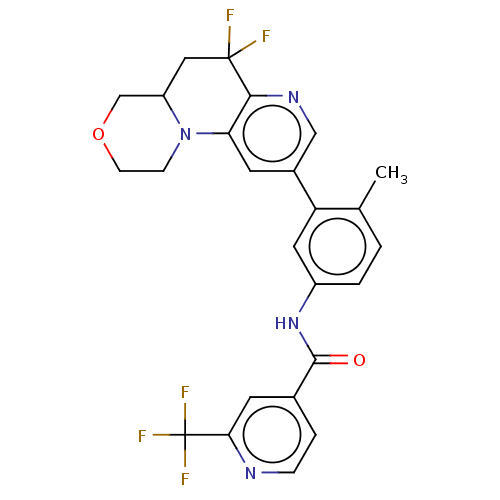 (US10377770, Example 111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410358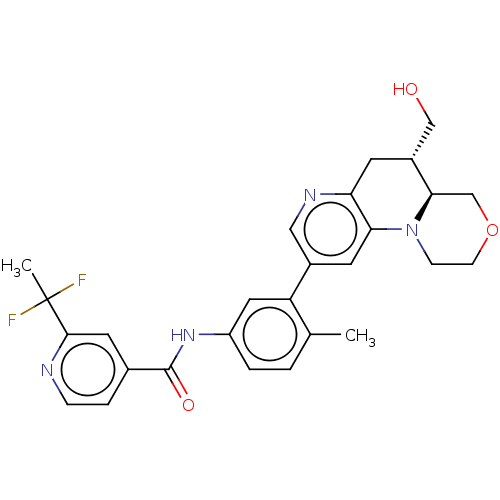 (2-(1,1-Difluoroethyl)-N-(3-((4aR,5R)-5-(hydroxymet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410393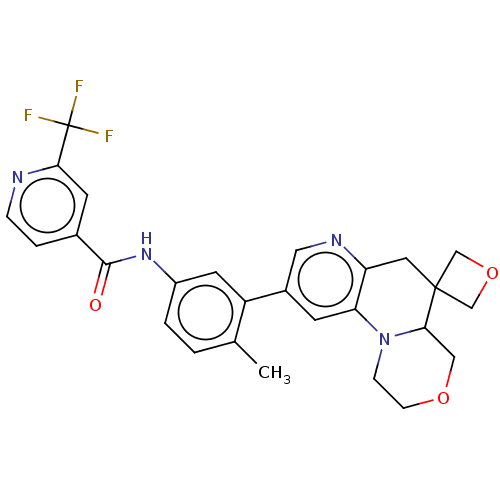 (US10377770, Example 153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410231 ((trans)-2-(1,1-difluoroethyl)-N-(3-(6-hydroxy-1,2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410306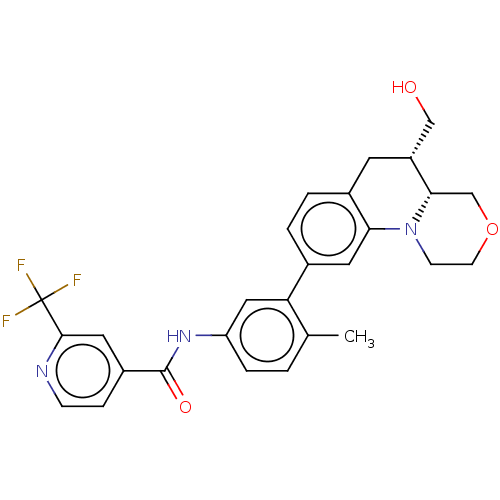 (2-(1,1-difluoroethyl)-N-(3-(5-(hydroxymethyl)-1,2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410349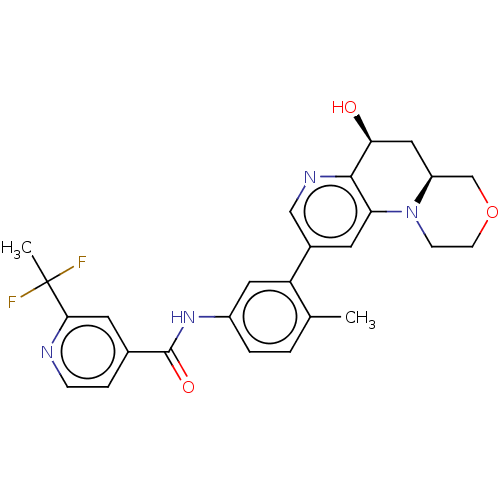 (US10377770, Example 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410293 (N-(3-(5,5-bis(hydroxymethyl)-1,2,4,4a,5,6-hexahydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410207 ((trans)-N-(3-(6-hydroxy-1,2,4,4a,5,6-hexahydro-[1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410399 (US10377770, Example 159) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410223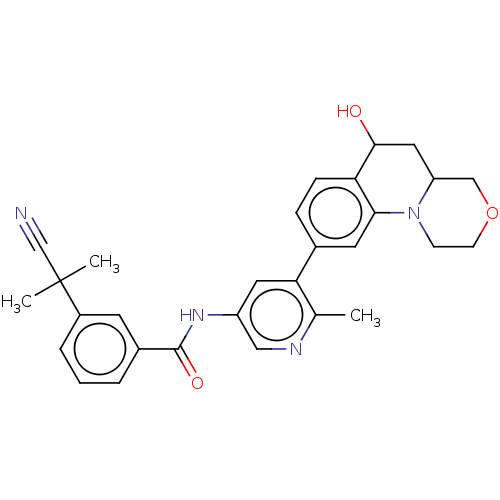 (US10377770, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410281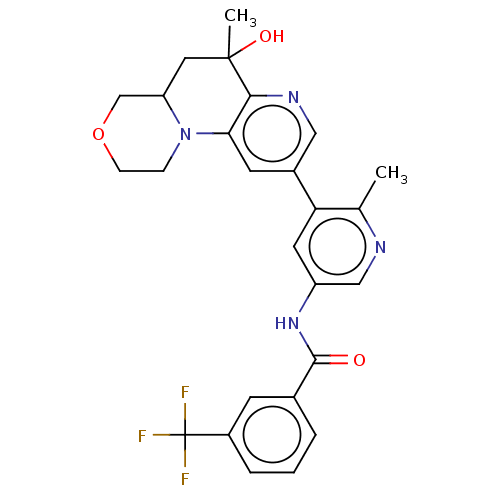 (US10377770, Example 43 | US10377770, Example 44) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410274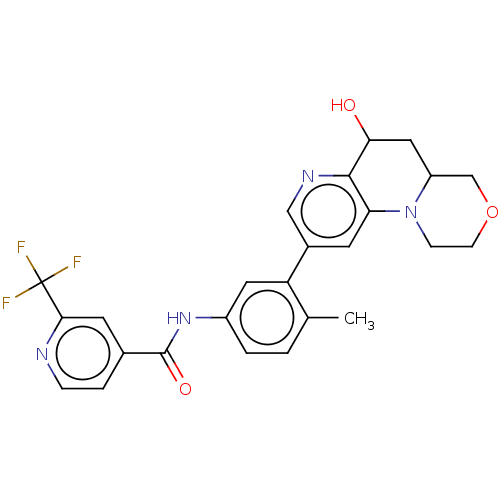 ((rac)-(cis)-N-(3-(6-hydroxy-1,2,4,4a,5,6-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410268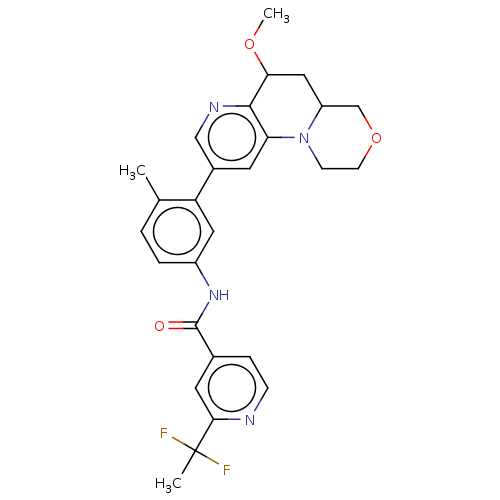 ((trans)-N-(3-(6-methoxy-1,2,4,4a,5,6-hexahydro-[1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410353 (US10377770, Example 113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340E,Y341E] (Homo sapiens (Human)) | BDBM410383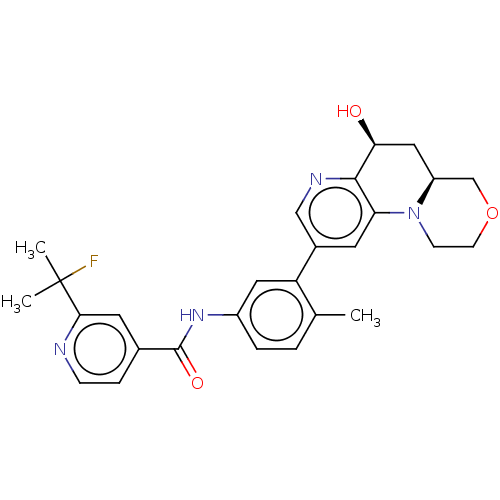 (US10377770, Example 143) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by well-known in vitro & in vivo methods. Raf inhibition data provided ... | US Patent US10377770 (2019) BindingDB Entry DOI: 10.7270/Q25X2C9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7392 total ) | Next | Last >> |