

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50109354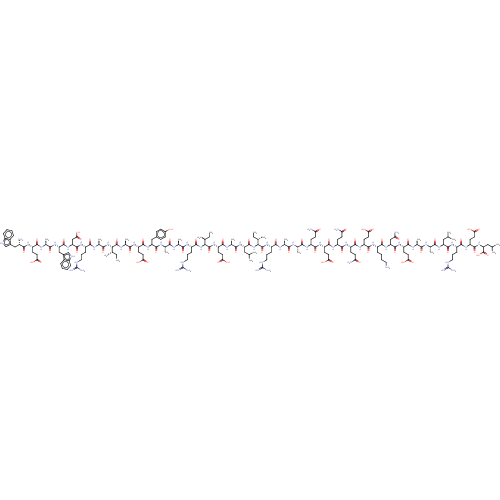 (CHEMBL3600949) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 gp41 N-terminal heptad repeat-mediated fusion between infected HL2/3 cells to target TZM-bl cells after 6 hrs by luciferase ... | J Med Chem 58: 6378-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00109 BindingDB Entry DOI: 10.7270/Q2HH6MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50109364 (CHEMBL529902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 gp41 N-terminal heptad repeat-mediated fusion between infected HL2/3 cells to target TZM-bl cells after 6 hrs by luciferase ... | J Med Chem 58: 6378-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00109 BindingDB Entry DOI: 10.7270/Q2HH6MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50109363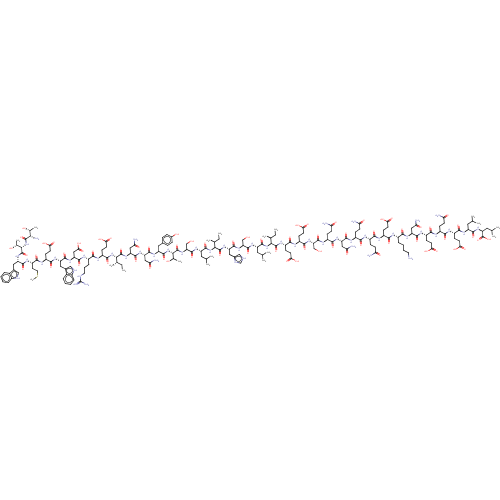 (CHEMBL3600950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 gp41 N-terminal heptad repeat-mediated fusion between infected HL2/3 cells to target TZM-bl cells after 6 hrs by luciferase ... | J Med Chem 58: 6378-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00109 BindingDB Entry DOI: 10.7270/Q2HH6MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50109355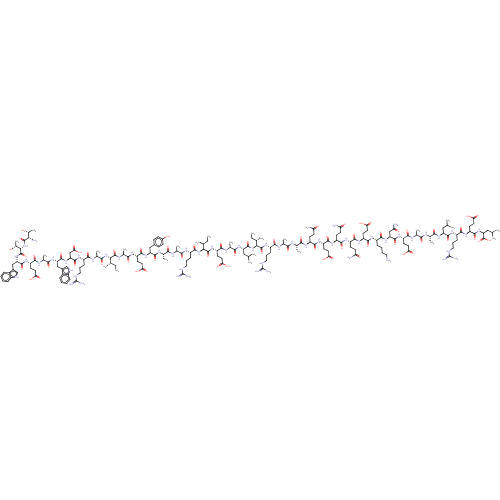 (CHEMBL428607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 gp41 N-terminal heptad repeat-mediated fusion between infected HL2/3 cells to target TZM-bl cells after 6 hrs by luciferase ... | J Med Chem 58: 6378-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00109 BindingDB Entry DOI: 10.7270/Q2HH6MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50109358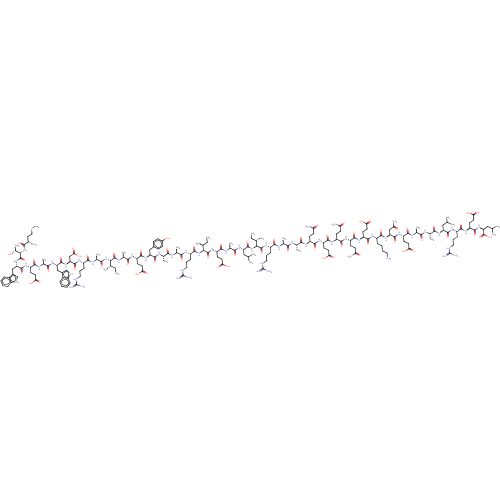 (CHEMBL411406) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 gp41 N-terminal heptad repeat-mediated fusion between infected HL2/3 cells to target TZM-bl cells after 6 hrs by luciferase ... | J Med Chem 58: 6378-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00109 BindingDB Entry DOI: 10.7270/Q2HH6MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461227 (US10774053, Compound 265 | US11352329, COMPD # 265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460936 (US10774053, Compound 49 | US11352329, COMPD # 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461185 (US10774053, Compound 235 | US11352329, COMPD # 235) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460932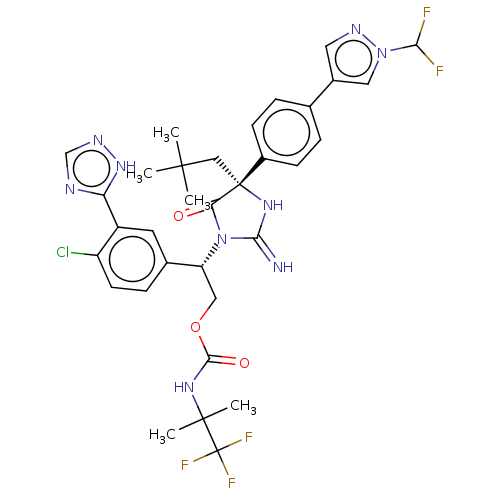 (US10774053, Compound 45 | US11352329, COMPD # 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460930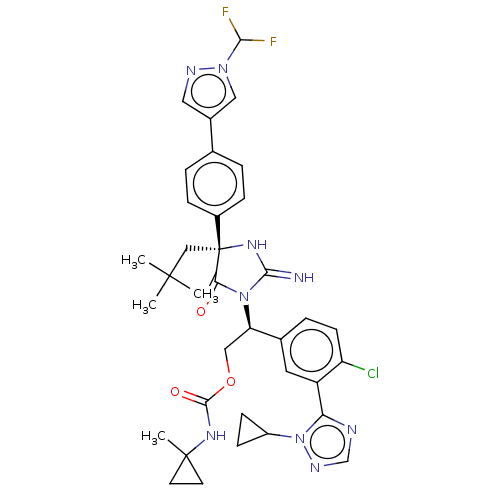 (US10774053, Compound 43 | US11352329, COMPD # 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460925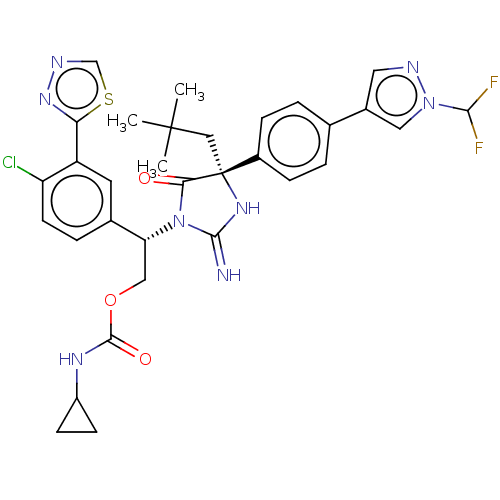 (US10774053, Compound 39 | US11352329, COMPD # 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460924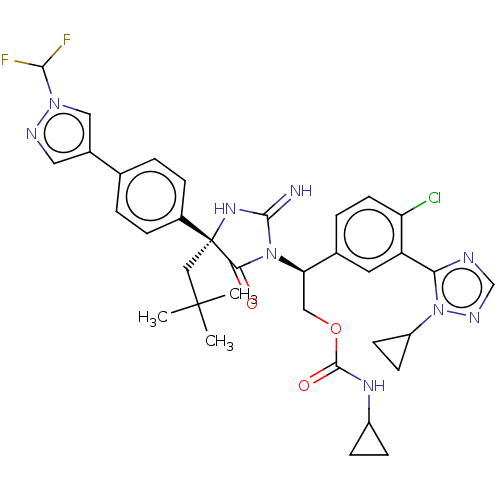 (US10774053, Compound 38 | US11352329, COMPD # 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460922 (US10774053, Compound 36 | US11352329, COMPD # 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460918 (US10774053, Compound 32 | US11352329, COMPD # 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460917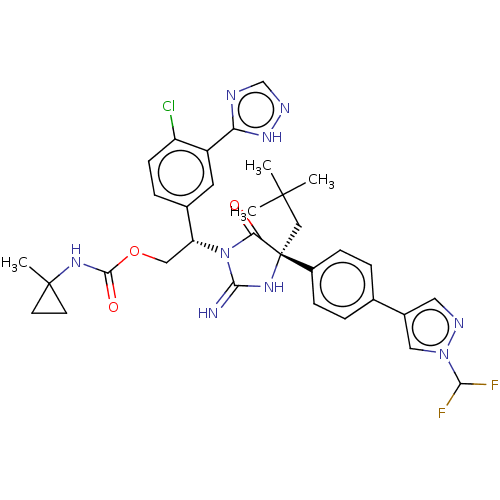 (US10774053, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460914 (US10774053, Compound 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460910 (US10774053, Compound 24 | US11352329, COMPD # 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460904 (US10774053, Compound 18 | US11352329, COMPD # 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460903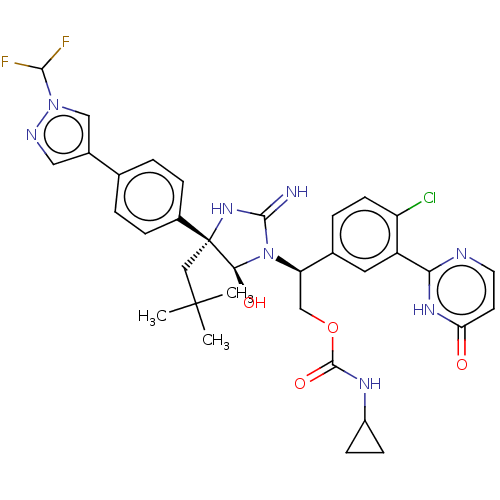 (US10774053, Compound 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460898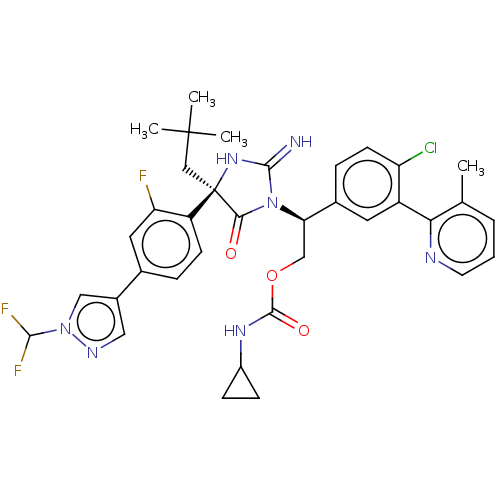 (US10774053, Compound 12 | US11352329, COMPD # 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460896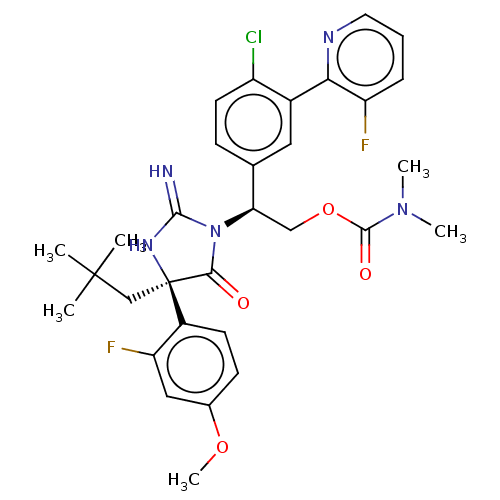 (US10774053, Compound 10 | US11352329, COMPD # 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460965 (US10774053, Compound 85 | US11352329, COMPD # 85) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461027 (US10774053, Compound 148 | US11352329, COMPD # 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461116 (US10774053, Compound 289 | US11352329, COMPD # 289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461115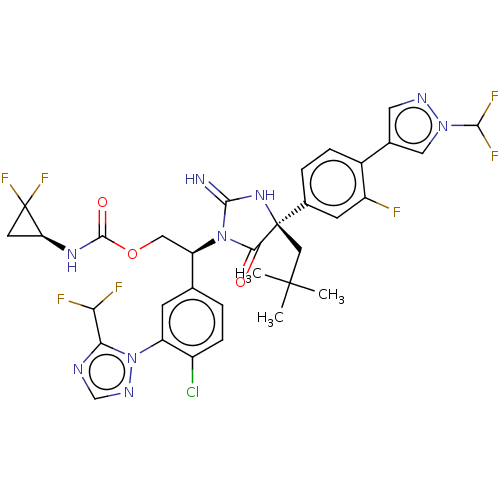 (US10774053, Compound 340 | US11352329, COMPD # 340) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461193 (US10774053, Compound 240 | US11352329, COMPD # 240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461194 (US10774053, Compound 241 | US11352329, COMPD # 241) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461208 (US10774053, Compound 252 | US11352329, COMPD # 252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461088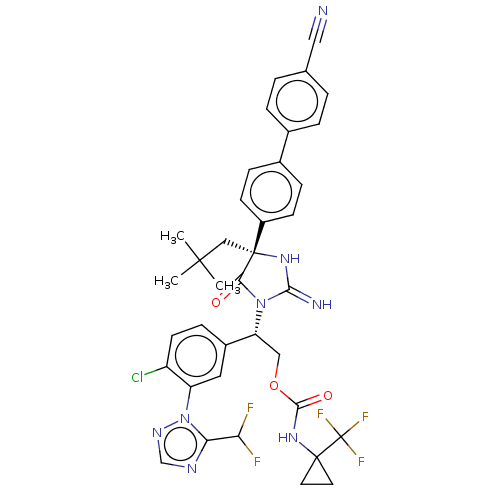 (US10774053, Compound 281 | US11352329, COMPD # 281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 45 mg/ml human ... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460967 (US10774053, Compound 87 | US11352329, COMPD # 87) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460974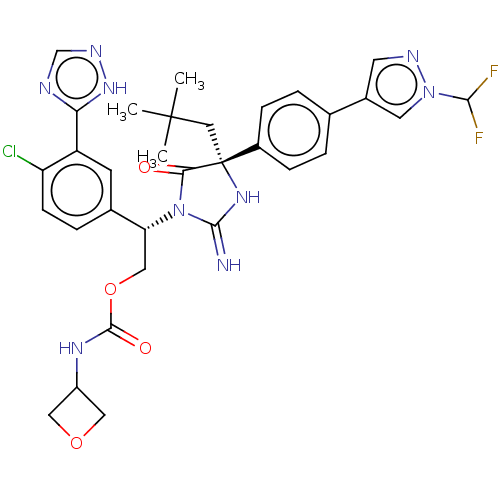 (US10774053, Compound 94 | US11352329, COMPD # 94) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461114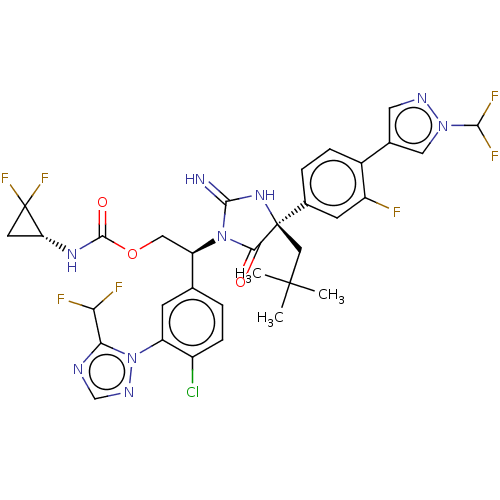 (US10774053, Compound 339 | US11352329, COMPD # 339) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460905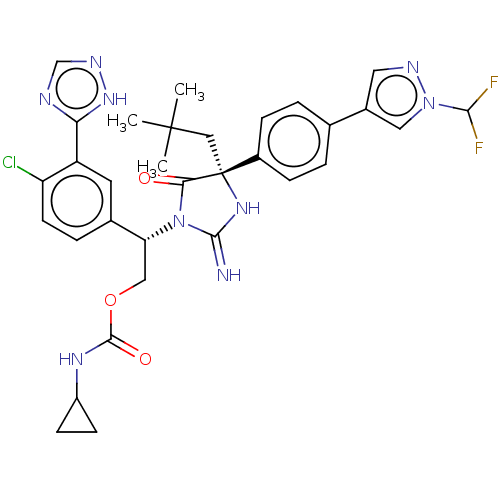 (US10774053, Compound 19 | US11352329, COMPD # 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460919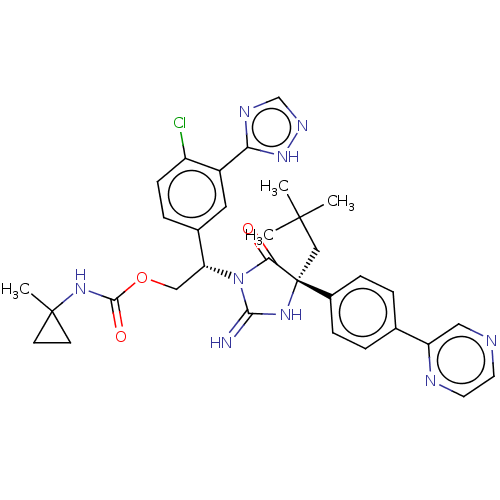 (US10774053, Compound 33 | US11352329, COMPD # 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460913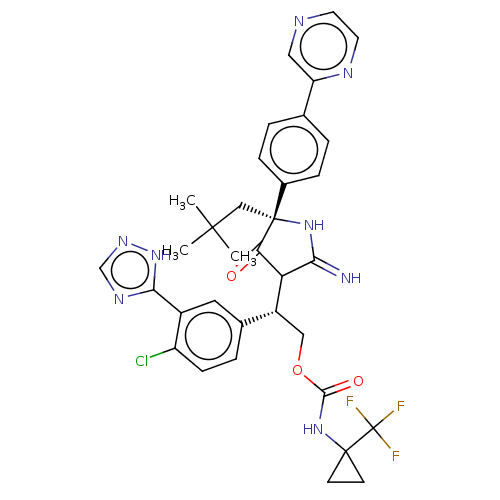 (US10774053, Compound 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461030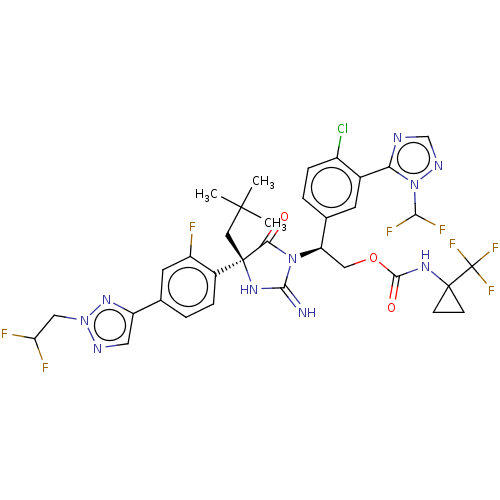 (US10774053, Compound 151 | US11352329, COMPD # 151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460927 (US10774053, Compound 41 | US11352329, COMPD # 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460961 (US10774053, Compound 81 | US11352329, COMPD # 81) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461052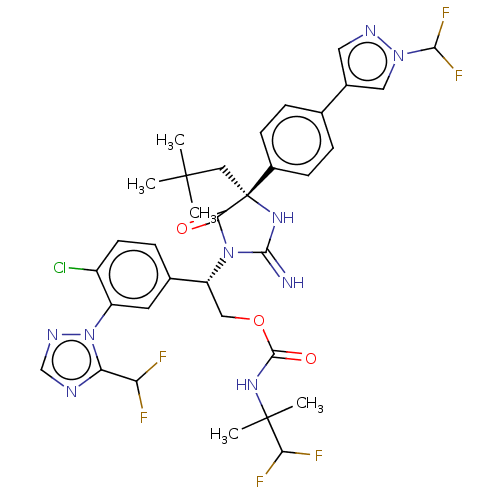 (US10774053, Compound 185 | US11352329, COMPD # 185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460972 (US10774053, Compound 92 | US11352329, COMPD # 92) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461043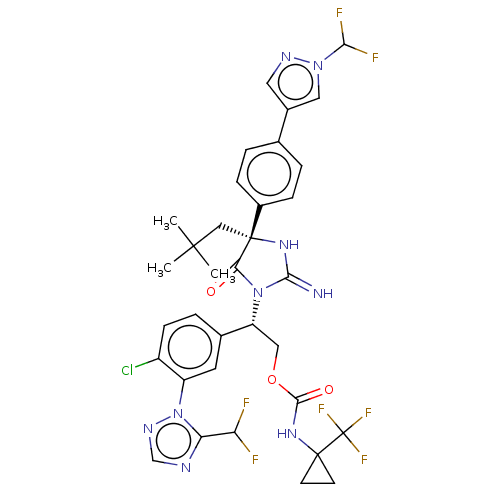 (US10774053, Compound 177 | US10774053, Compound 25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461044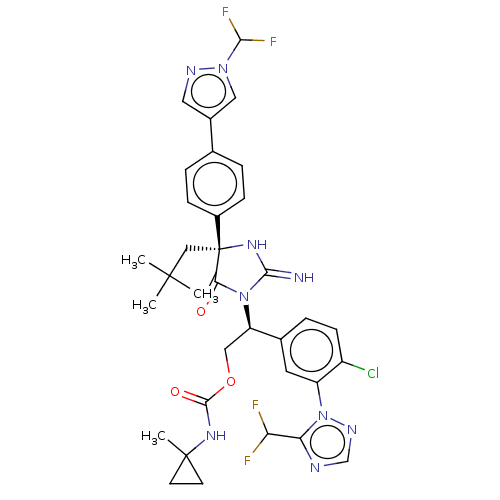 (US10774053, Compound 178 | US11352329, COMPD # 178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461195 (US10774053, Compound 242 | US11352329, COMPD # 242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM461186 (US10774053, Compound 236 | US11352329, COMPD # 236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of alpha-acid glyc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 1 mg/ml alpha-a... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460984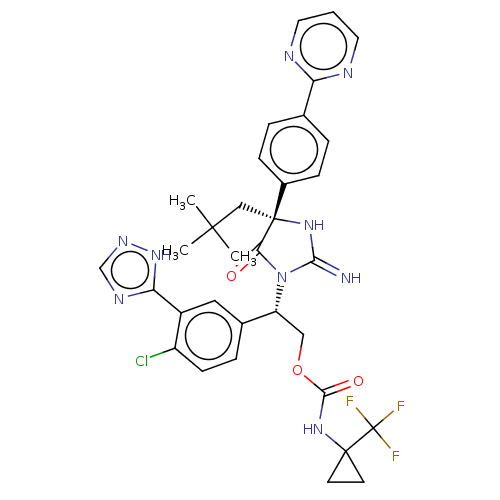 (US10774053, Compound 105 | US11352329, COMPD # 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM460989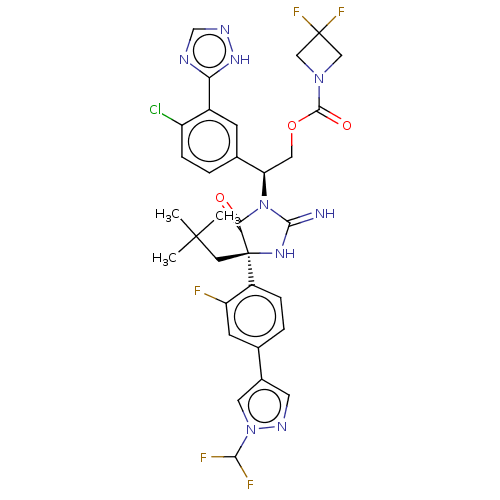 (US10774053, Compound 111 | US11352329, COMPD # 111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in a high-throughput 384-well assay format for their ability to inhibit the replication of HIV-1 (IIIB) in MT-4 cells. Compound... | US Patent US10774053 (2020) BindingDB Entry DOI: 10.7270/Q2DV1NX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1130 total ) | Next | Last >> |