 Found 115 hits for UniProtKB: Q9BPZ7
Found 115 hits for UniProtKB: Q9BPZ7 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114039
BindingDB Entry DOI: 10.7270/Q2TT4VZR |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606735
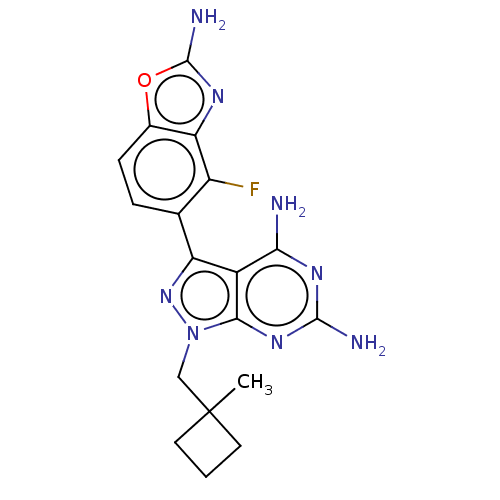
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50503131
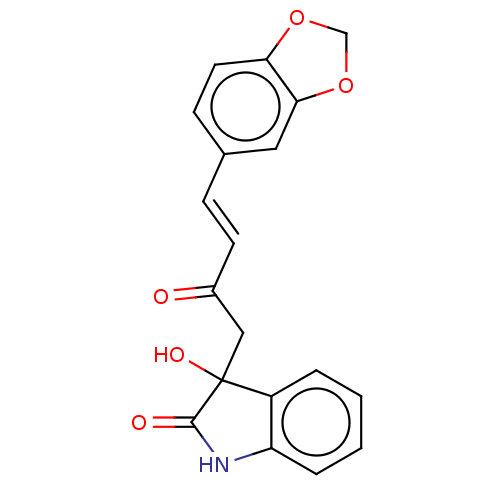
(CHEMBL4563142)Show SMILES OC1(CC(=O)\C=C\c2ccc3OCOc3c2)C(=O)Nc2ccccc12 Show InChI InChI=1S/C19H15NO5/c21-13(7-5-12-6-8-16-17(9-12)25-11-24-16)10-19(23)14-3-1-2-4-15(14)20-18(19)22/h1-9,23H,10-11H2,(H,20,22)/b7-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606737
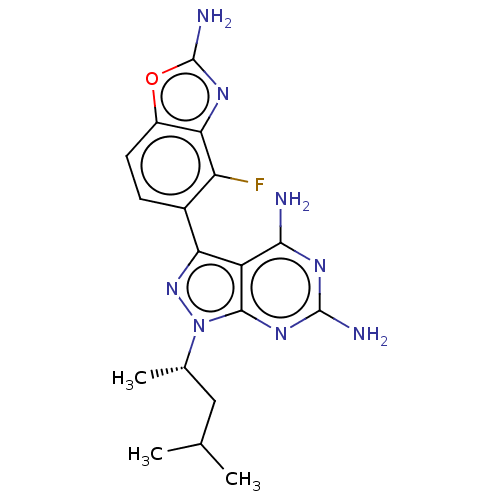
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606736
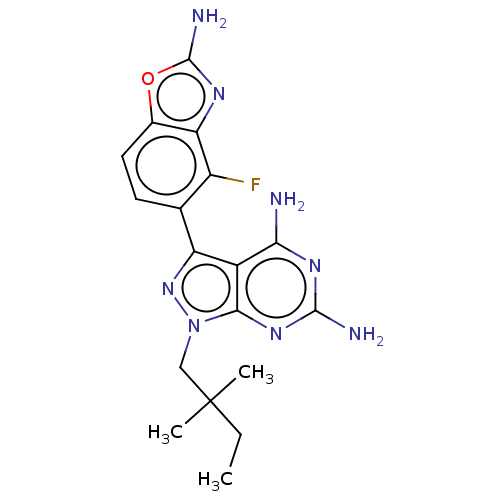
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606741
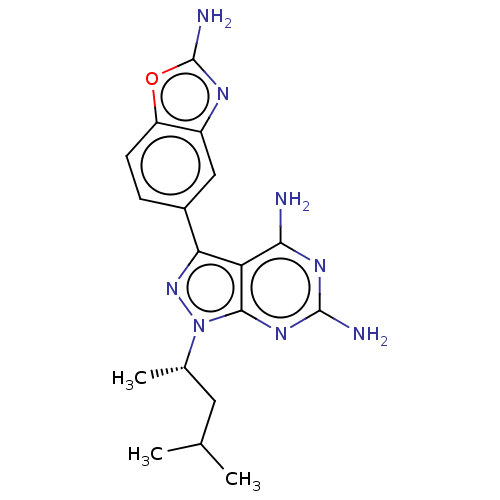
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606733

(CHEMBL5218988) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606739
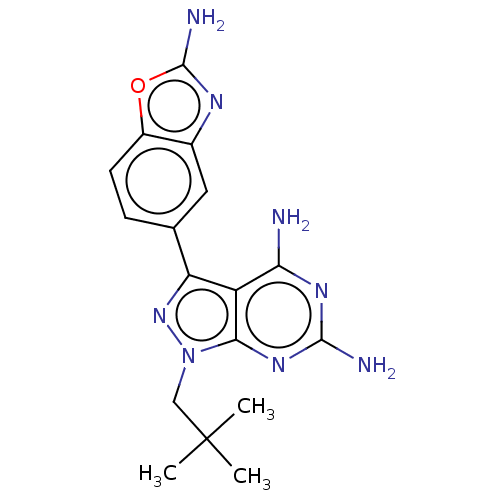
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606740
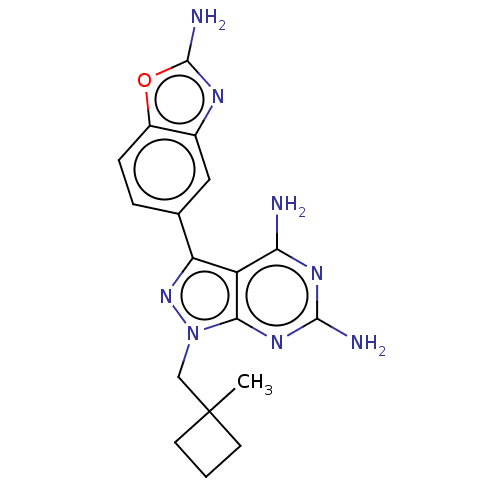
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606742
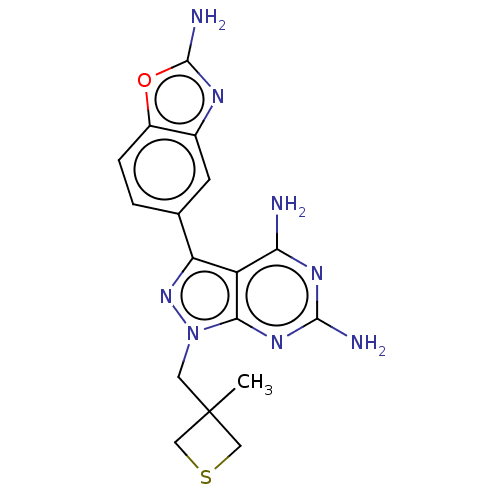
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50348452
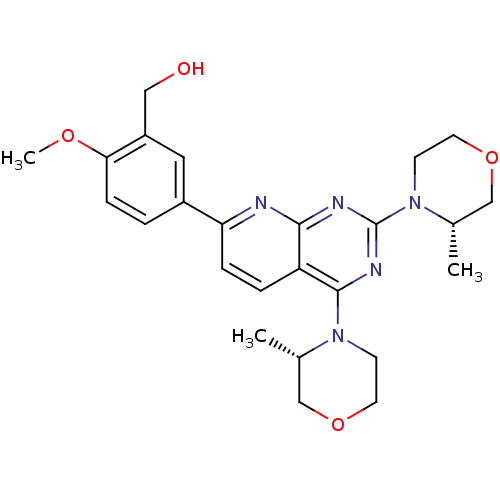
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM315477
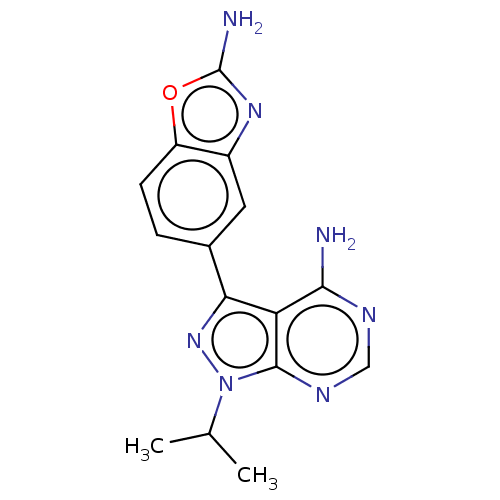
(US10172858, Table 1.1 | US10172858, Table 1.22)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM35587
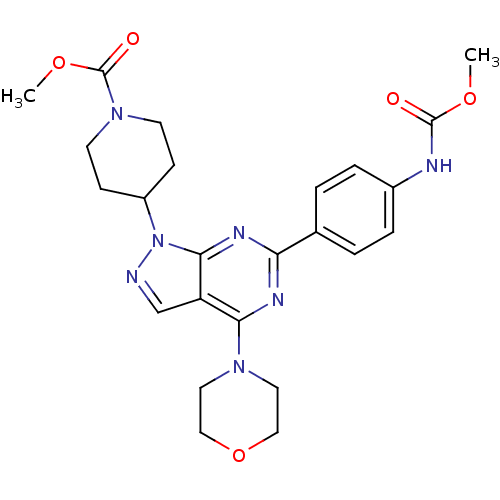
(pyrazolo pyrimidine, 19)Show SMILES COC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(C3CCN(CC3)C(=O)OC)c2n1 Show InChI InChI=1S/C24H29N7O5/c1-34-23(32)26-17-5-3-16(4-6-17)20-27-21(29-11-13-36-14-12-29)19-15-25-31(22(19)28-20)18-7-9-30(10-8-18)24(33)35-2/h3-6,15,18H,7-14H2,1-2H3,(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529393

(CHEMBL4564479 | US11731973, Example 24)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-13-9(6-18-14(16)20-13)12(21-22)8-3-4-11-10(5-8)19-15(17)23-11/h3-7H,1-2H3,(H2,17,19)(H2,16,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606738
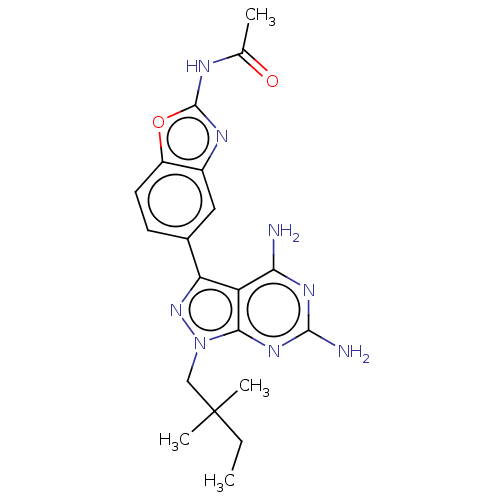
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM582479

(5-(2-aminobenzoxazol-5-yl)-7-methyl-7-methylsulfan...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Rapamycin-insensitive companion of mTOR/Serine/threonine-protein kinase mTOR/Target of rapamycin complex 2 subunit MAPKAP1/Target of rapamycin complex subunit LST8
(Homo sapiens (Human)) | BDBM50310989
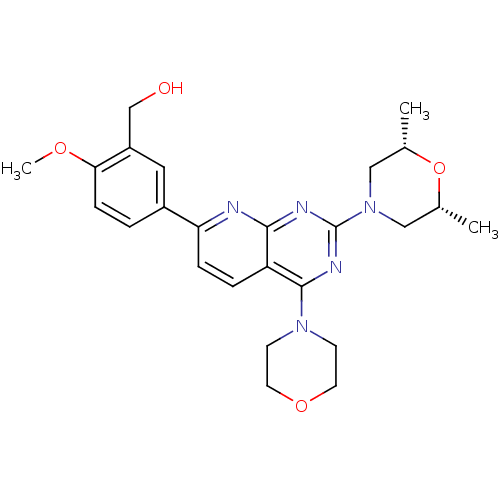
((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in HEK293 cells using GST-tagged S6K1 or Akt1 as substrate after 30 mins by immunoblotting assay |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50328584
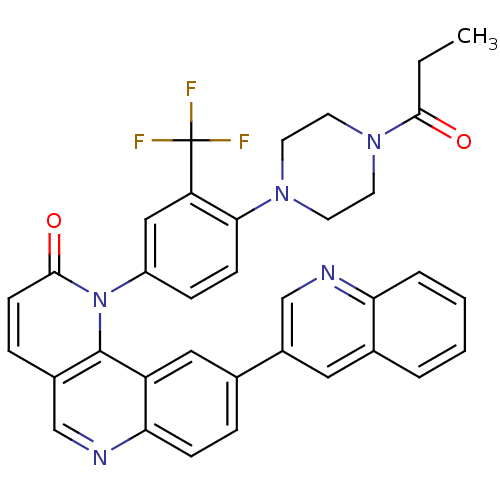
(1-(4-(4-Propionylpiperazin-1-yl)-3-(trifluoromethy...)Show SMILES CCC(=O)N1CCN(CC1)c1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 Show InChI InChI=1S/C35H28F3N5O2/c1-2-32(44)42-15-13-41(14-16-42)31-11-9-26(19-28(31)35(36,37)38)43-33(45)12-8-24-20-40-30-10-7-22(18-27(30)34(24)43)25-17-23-5-3-4-6-29(23)39-21-25/h3-12,17-21H,2,13-16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally FLAG-tagged mTORC2 (unknown origin) expressed in human HeLa cells using S6K1 or Akt1 as substrate after 20 mins by immunob... |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50341209
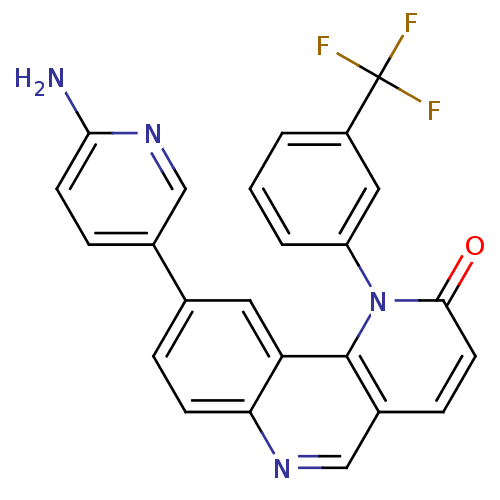
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminally FLAG-tagged mTORC2 (unknown origin) expressed in human HeLa cells using S6K1 or Akt1 as substrate after 20 mins by immunob... |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529395

(CHEMBL4529672 | US11731973, Example 21)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 Show InChI InChI=1S/C15H16N8O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H4,16,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM582478

(5-(2-aminobenzoxazol-5-yl)-7-isobutyl-6, 7-dihydro...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50535043
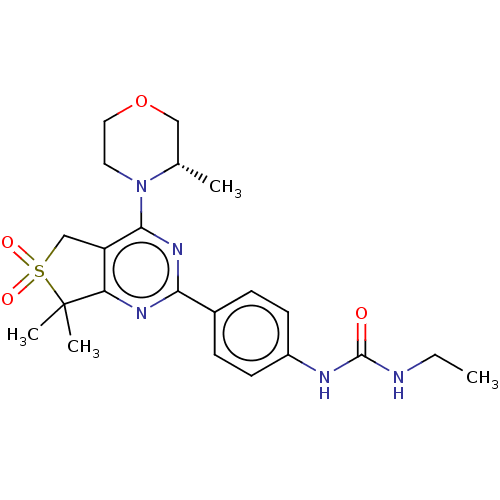
(CHEMBL4577549)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CS(=O)(=O)C2(C)C)c(n1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C22H29N5O4S/c1-5-23-21(28)24-16-8-6-15(7-9-16)19-25-18-17(13-32(29,30)22(18,3)4)20(26-19)27-10-11-31-12-14(27)2/h6-9,14H,5,10-13H2,1-4H3,(H2,23,24,28)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in HEK293T/17 cells assessed as reduction in Akt phosphorylation at Ser-473 residue after 2 hrs by sandwich immunoassay |
ACS Med Chem Lett 7: 768-73 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00149
BindingDB Entry DOI: 10.7270/Q2HH6PKM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606734

(CHEMBL5221072) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50546183
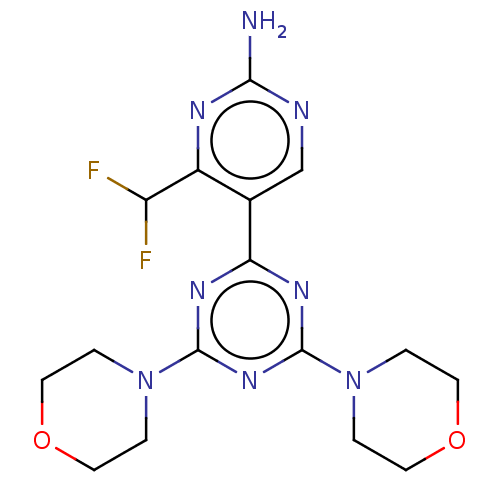
(CHEMBL4744705)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residue incubated for 1 hr by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00333
BindingDB Entry DOI: 10.7270/Q2VT1WPR |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529398
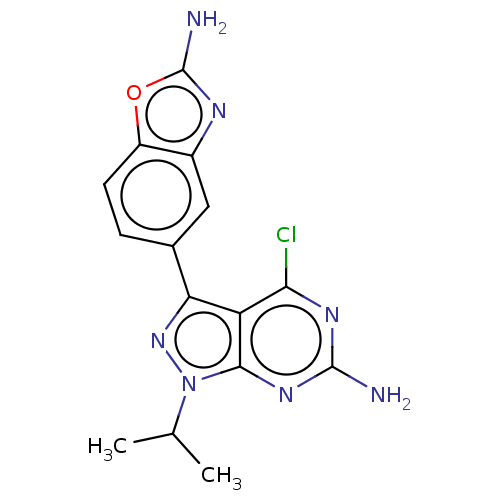
(CHEMBL4549761 | US11731973, Example 31)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C15H14ClN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529407

(CHEMBL4572049)Show SMILES CCCCn1nc(-c2ccc3oc(N)nc3c2)c2c(Cl)nc(N)nc12 Show InChI InChI=1S/C16H16ClN7O/c1-2-3-6-24-14-11(13(17)21-15(18)22-14)12(23-24)8-4-5-10-9(7-8)20-16(19)25-10/h4-5,7H,2-3,6H2,1H3,(H2,19,20)(H2,18,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606762
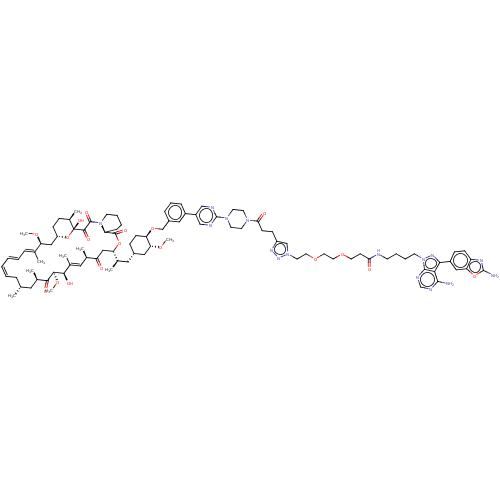
(CHEMBL5218550)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCc2cccc(c2)-c2cnc(nc2)N2CCN(CC2)C(=O)CCc2cn(CCOCCOCCC(=O)NCCCCn3nc(-c4ccc5nc(N)oc5c4)c4c(N)ncnc34)nn2)[C@@H](C1)OC |r,c:31,47,51,t:49| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606763
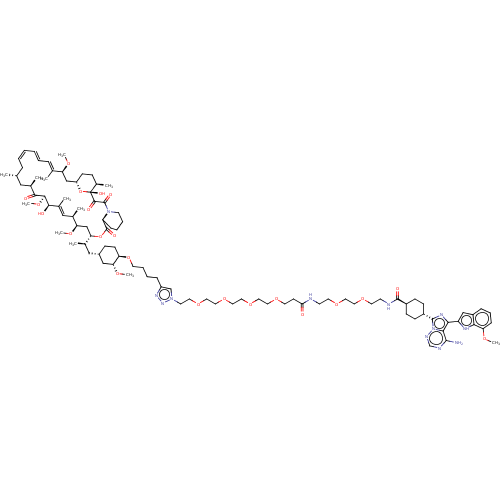
(CHEMBL5219551)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(C[C@@H](OC)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCCCc2cn(CCOCCOCCOCCOCCC(=O)NCCOCCOCCNC(=O)[C@H]3CC[C@@H](CC3)c3nc(-c4cc5cccc(OC)c5[nH]4)c4c(N)ncnn34)nn2)[C@@H](C1)OC |r,wU:18.20,57.60,60.62,129.140,22.24,35.37,40.42,1.0,4.4,6.6,25.27,103.106,wD:63.66,28.30,43.45,53.57,33.35,100.102,c:32,48,52,t:50,(-26.38,-7.52,;-27.6,-6.59,;-28.89,-5.74,;-28.77,-4.28,;-27.41,-3.59,;-27.33,-2.05,;-26.16,-4.36,;-25.96,-2.83,;-26.24,-5.9,;-24.88,-3.71,;-24.76,-2.25,;-23.59,-4.55,;-23.71,-6.01,;-22.31,-3.91,;-22.24,-2.37,;-20.87,-1.67,;-19.58,-2.52,;-19.7,-3.97,;-21.07,-4.67,;-19.85,-5.61,;-18.43,-5.02,;-20.05,-7.13,;-18.83,-8.07,;-17.61,-9.01,;-19.03,-9.6,;-17.81,-10.54,;-16.39,-9.95,;-15.17,-10.89,;-18.1,-12.05,;-16.93,-13.05,;-19.55,-12.56,;-19.83,-14.08,;-18.66,-15.08,;-21.29,-14.59,;-22.45,-13.58,;-21.57,-16.1,;-20.4,-17.1,;-20.69,-18.62,;-22.7,-17.7,;-22.12,-19.12,;-24.42,-17.77,;-25.01,-16.35,;-25.36,-18.99,;-26.89,-18.79,;-27.82,-20.01,;-27.47,-17.37,;-29,-17.16,;-29.59,-15.74,;-28.65,-14.52,;-29.24,-13.1,;-28.3,-11.87,;-28.89,-10.45,;-30.42,-10.25,;-27.95,-9.23,;-28.54,-7.81,;-26.42,-9.43,;-25.49,-8.21,;-17.41,-7.48,;-16.19,-8.42,;-17.2,-5.96,;-15.78,-5.37,;-14.56,-6.31,;-13.14,-5.72,;-12.93,-4.19,;-11.51,-3.6,;-10.29,-4.54,;-8.87,-3.95,;-7.64,-4.89,;-7.85,-6.42,;-6.63,-7.36,;-5.11,-6.86,;-4.24,-8.13,;-2.71,-8.32,;-1.78,-7.1,;-.25,-7.29,;.68,-6.07,;2.21,-6.26,;3.14,-5.04,;4.67,-5.23,;5.6,-4.01,;7.13,-4.2,;8.06,-2.98,;9.59,-3.17,;10.52,-1.95,;12.05,-2.14,;12.98,-.92,;14.51,-1.11,;15.11,-2.53,;15.44,.11,;16.97,-.08,;17.9,1.14,;19.43,.95,;20.36,2.18,;21.89,1.98,;22.82,3.21,;24.35,3.01,;25.28,4.24,;26.81,4.04,;27.74,5.27,;29.27,5.07,;27.15,6.69,;28.08,7.91,;27.49,9.33,;25.96,9.53,;25.03,8.3,;25.62,6.88,;25.36,10.95,;26.16,12.26,;25.16,13.43,;25.51,14.93,;24.51,16.1,;25.31,17.41,;24.86,18.89,;25.92,20.01,;27.42,19.66,;27.86,18.18,;29.36,17.83,;30.42,18.95,;26.81,17.06,;26.93,15.53,;23.74,12.84,;22.35,13.49,;22.08,15.01,;21.08,12.62,;21.21,11.08,;22.6,10.42,;23.86,11.3,;-5.17,-9.35,;-6.62,-8.84,;-14.16,-3.25,;-15.58,-3.84,;-13.95,-1.73,;-15.17,-.79,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606764
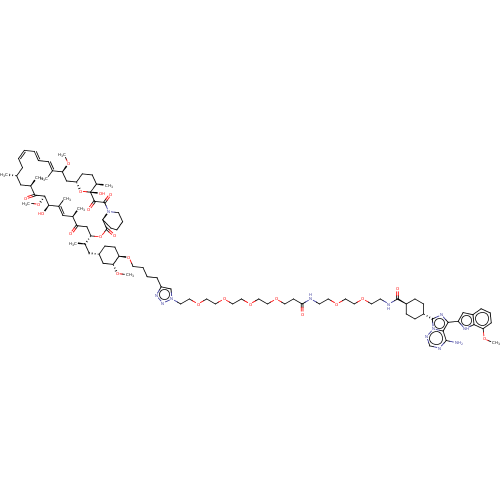
(CHEMBL5220568)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCCCc2cn(CCOCCOCCOCCOCCC(=O)NCCOCCOCCNC(=O)[C@H]3CC[C@@H](CC3)c3nc(-c4cc5cccc(OC)c5[nH]4)c4c(N)ncnn34)nn2)[C@@H](C1)OC |wU:1.0,59.61,128.139,56.59,18.20,22.24,34.36,39.41,4.4,6.6,102.108,wD:62.65,27.29,32.34,42.44,52.56,99.101,c:31,47,51,t:49,(-27.88,-14.45,;-29.37,-14.85,;-30.83,-15.36,;-31.91,-14.37,;-31.63,-12.87,;-32.81,-11.87,;-30.27,-12.35,;-31.36,-11.26,;-29.09,-13.35,;-30,-10.93,;-31.09,-9.95,;-28.55,-10.43,;-27.47,-11.41,;-28.28,-9.02,;-29.46,-8.02,;-29.18,-6.51,;-27.72,-6.01,;-26.64,-6.99,;-26.92,-8.5,;-25.44,-8.1,;-25.04,-6.61,;-24.35,-9.19,;-22.86,-8.79,;-21.37,-8.39,;-21.77,-9.88,;-20.28,-9.48,;-19.88,-7.99,;-19.25,-10.63,;-17.75,-10.31,;-19.73,-12.09,;-18.7,-13.24,;-17.2,-12.92,;-19.18,-14.7,;-20.69,-15.02,;-18.15,-15.85,;-16.65,-15.53,;-15.62,-16.68,;-17.57,-17.72,;-16.09,-18.12,;-18.56,-19.13,;-20.05,-18.73,;-18.16,-20.61,;-19.25,-21.7,;-18.85,-23.19,;-20.74,-21.3,;-21.83,-22.39,;-23.32,-21.99,;-23.71,-20.51,;-25.2,-20.11,;-25.6,-18.62,;-27.09,-18.22,;-28.18,-19.31,;-27.49,-16.73,;-28.97,-16.34,;-26.4,-15.65,;-26.8,-14.16,;-22.46,-7.3,;-20.97,-6.9,;-23.55,-6.21,;-23.15,-4.72,;-21.66,-4.33,;-21.26,-2.84,;-22.35,-1.75,;-21.95,-.26,;-20.47,.14,;-20.07,1.63,;-18.58,2.02,;-18.18,3.51,;-16.69,3.91,;-16.14,5.35,;-14.61,5.27,;-13.52,6.35,;-12.03,5.96,;-10.94,7.04,;-9.45,6.65,;-8.36,7.74,;-6.88,7.34,;-5.79,8.43,;-4.3,8.03,;-3.21,9.12,;-1.72,8.72,;-.64,9.81,;.85,9.41,;1.94,10.5,;3.43,10.1,;4.52,11.19,;4.12,12.67,;6,10.79,;7.09,11.88,;8.58,11.48,;9.67,12.57,;11.16,12.17,;12.25,13.26,;13.73,12.86,;14.82,13.95,;16.31,13.55,;17.4,14.64,;18.89,14.24,;19.28,12.75,;19.98,15.33,;21.46,14.93,;22.55,16.02,;22.15,17.51,;20.67,17.91,;19.58,16.82,;23.24,18.6,;24.76,18.35,;25.46,19.73,;26.95,20.12,;27.5,21.56,;29.04,21.48,;30.13,22.57,;31.61,22.17,;32.01,20.68,;30.92,19.6,;31.32,18.11,;32.81,17.71,;29.44,19.99,;28.14,19.16,;24.37,20.81,;24.45,22.35,;25.79,23.12,;23.16,23.19,;21.79,22.49,;21.71,20.95,;23,20.12,;-14.21,3.78,;-15.5,2.94,;-23.84,-2.15,;-24.24,-3.64,;-24.93,-1.06,;-26.42,-1.46,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
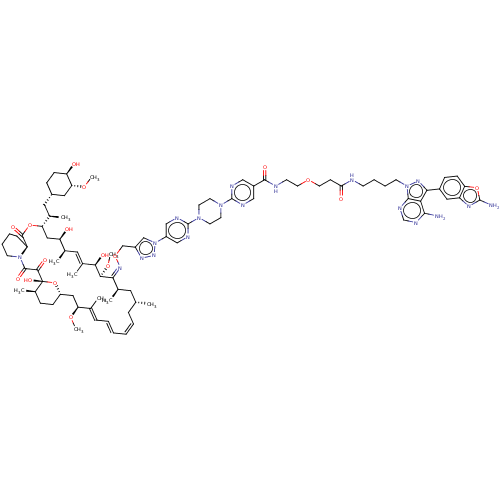
(Homo sapiens) | BDBM50606765
(CHEMBL5219300)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(C[C@@H](O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)\C(=N/OCc1cn(nn1)-c1cnc(nc1)N1CCN(CC1)c1ncc(cn1)C(=O)NCCOCCC(=O)NCCCCn1nc(-c3ccc4oc(N)nc4c3)c3c(N)ncnc13)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:31,115,119,t:117| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
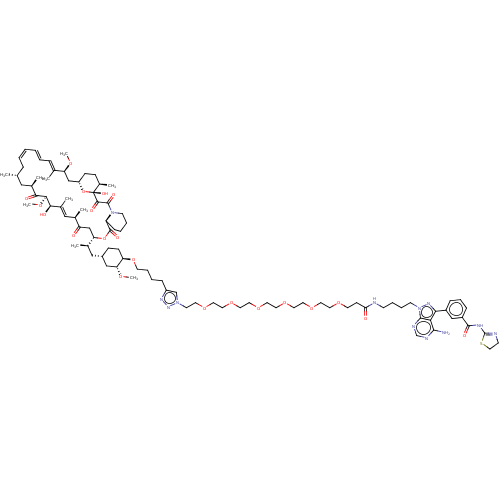
(Homo sapiens) | BDBM50606761
(CHEMBL5220263)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCCCc2cn(CCOCCOCCOCCOCCOCCOCCC(=O)NCCCCn3nc(-c4cccc(c4)C(=O)NC4=NCCS4)c4c(N)ncnc34)nn2)[C@@H](C1)OC |c:31,47,51,t:49,114| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50546183

(CHEMBL4744705)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
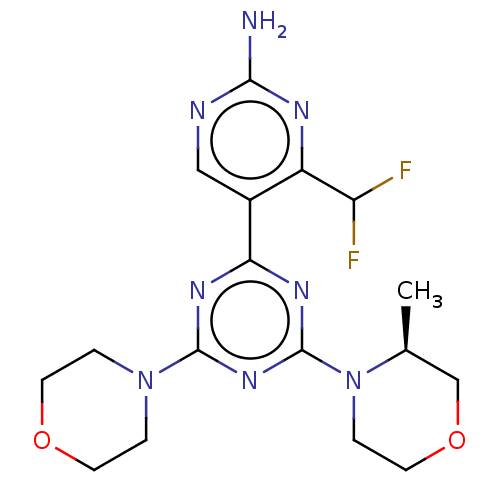
(Homo sapiens) | BDBM50515198
(CHEMBL4445573)Show SMILES C[C@H]1COCCN1c1nc(nc(n1)-c1cnc(N)nc1C(F)F)N1CCOCC1 |r| Show InChI InChI=1S/C17H22F2N8O2/c1-10-9-29-7-4-27(10)17-24-14(11-8-21-15(20)22-12(11)13(18)19)23-16(25-17)26-2-5-28-6-3-26/h8,10,13H,2-7,9H2,1H3,(H2,20,21,22)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50431522

(CHEMBL2348865)Show SMILES O=c1[nH]c2ncc(nc2n1CCC1CCOCC1)-c1ccc(cc1)-c1nnc[nH]1 Show InChI InChI=1S/C20H21N7O2/c28-20-25-18-19(27(20)8-5-13-6-9-29-10-7-13)24-16(11-21-18)14-1-3-15(4-2-14)17-22-12-23-26-17/h1-4,11-13H,5-10H2,(H,21,25,28)(H,22,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50431521

(CHEMBL2348864)Show SMILES O=C1CN(CCC2CCOCC2)c2nc(cnc2N1)-c1ccc(cc1)-c1nnc[nH]1 Show InChI InChI=1S/C21H23N7O2/c29-18-12-28(8-5-14-6-9-30-10-7-14)21-20(26-18)22-11-17(25-21)15-1-3-16(4-2-15)19-23-13-24-27-19/h1-4,11,13-14H,5-10,12H2,(H,22,26,29)(H,23,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606704
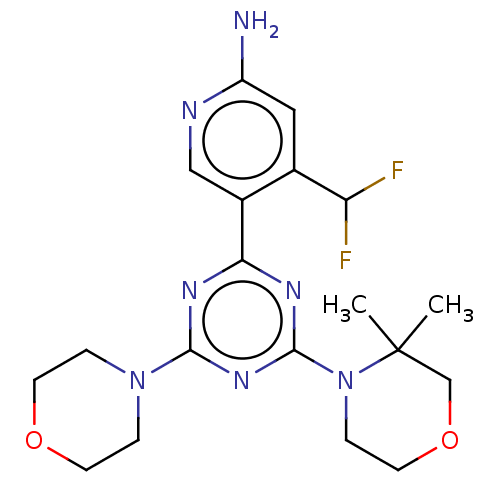
(CHEMBL5218488)Show SMILES CC1(C)COCCN1c1nc(nc(n1)-c1cnc(N)cc1C(F)F)N1CCOCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
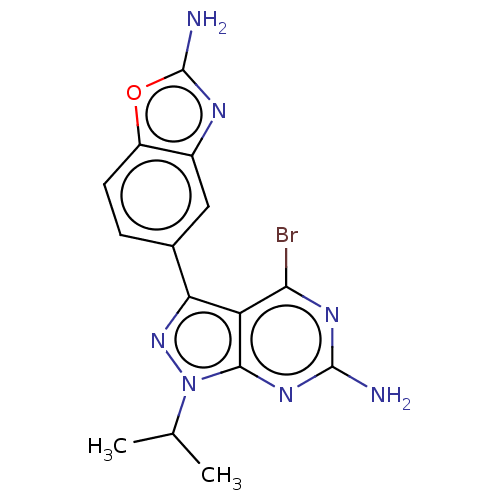
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529405
(CHEMBL4588610)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(Br)nc(N)nc12 Show InChI InChI=1S/C15H14BrN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50546184
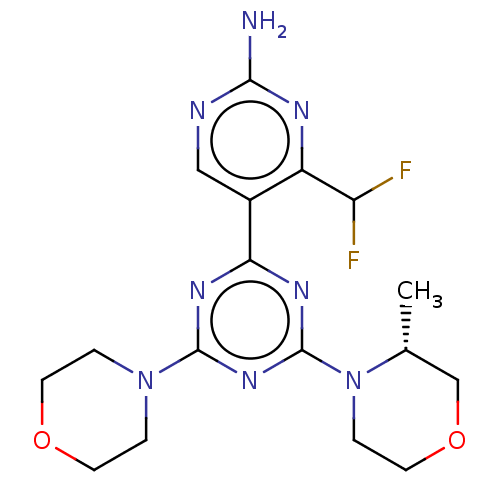
(CHEMBL4758284)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1cnc(N)nc1C(F)F)N1CCOCC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residue incubated for 1 hr by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00333
BindingDB Entry DOI: 10.7270/Q2VT1WPR |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50546185

(CHEMBL4749136)Show SMILES [H][C@]12CC[C@]([H])(COC1)N2c1nc(nc(n1)-c1cnc(N)nc1C(F)F)N1CCOCC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residue incubated for 1 hr by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00333
BindingDB Entry DOI: 10.7270/Q2VT1WPR |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50515201

(CHEMBL4453693)Show SMILES C[C@H]1COCCN1c1nc(nc(n1)-c1cnc(N)cc1C(F)F)N1CCOCC1 |r| Show InChI InChI=1S/C18H23F2N7O2/c1-11-10-29-7-4-27(11)18-24-16(13-9-22-14(21)8-12(13)15(19)20)23-17(25-18)26-2-5-28-6-3-26/h8-9,11,15H,2-7,10H2,1H3,(H2,21,22)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50505407
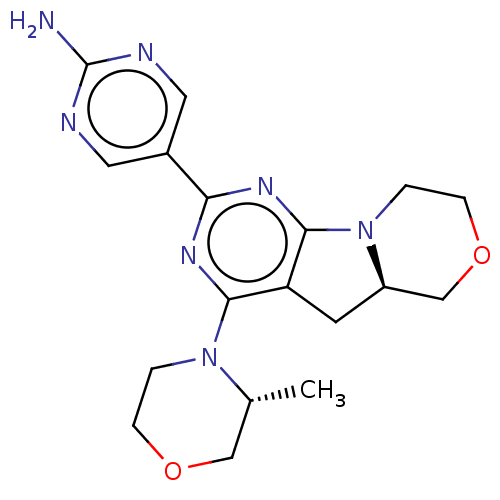
(CHEMBL4436124)Show SMILES [H][C@]12Cc3c(nc(nc3N3CCOC[C@H]3C)-c3cnc(N)nc3)N1CCOC2 |r| Show InChI InChI=1S/C18H23N7O2/c1-11-9-26-4-2-24(11)16-14-6-13-10-27-5-3-25(13)17(14)23-15(22-16)12-7-20-18(19)21-8-12/h7-8,11,13H,2-6,9-10H2,1H3,(H2,19,20,21)/t11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residues incubated for 1 hr by Western blot analysis |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972
BindingDB Entry DOI: 10.7270/Q2DJ5JW1 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529399
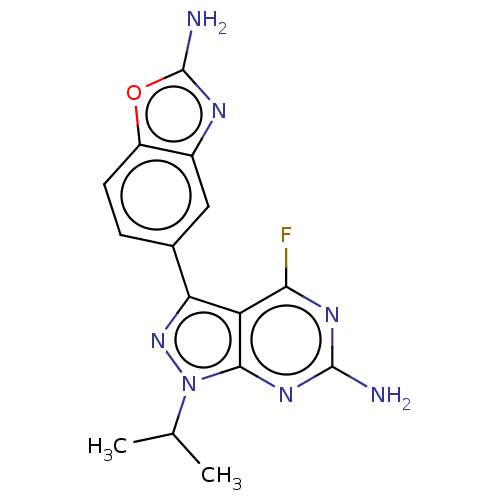
(CHEMBL4538004)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c(F)nc(N)nc12 Show InChI InChI=1S/C15H14FN7O/c1-6(2)23-13-10(12(16)20-14(17)21-13)11(22-23)7-3-4-9-8(5-7)19-15(18)24-9/h3-6H,1-2H3,(H2,18,19)(H2,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50503139
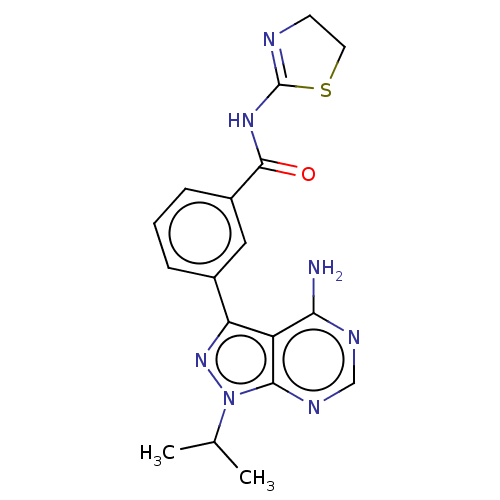
(CHEMBL1242115)Show SMILES CC(C)n1nc(-c2cccc(c2)C(=O)NC2=NCCS2)c2c(N)ncnc12 |t:16| Show InChI InChI=1S/C18H19N7OS/c1-10(2)25-16-13(15(19)21-9-22-16)14(24-25)11-4-3-5-12(8-11)17(26)23-18-20-6-7-27-18/h3-5,8-10H,6-7H2,1-2H3,(H2,19,21,22)(H,20,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM36409
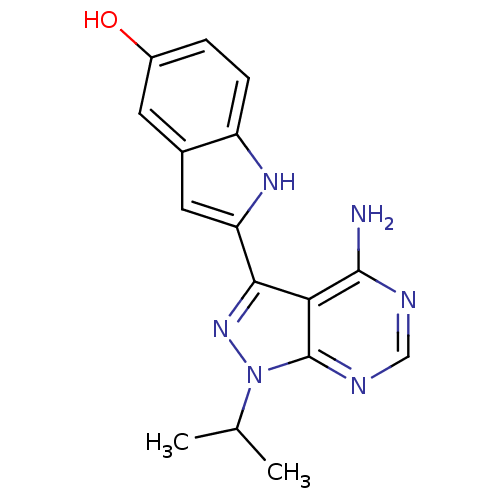
(2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin...)Show SMILES CC(C)n1nc(-c2cc3cc(O)ccc3[nH]2)c2c(N)ncnc12 Show InChI InChI=1S/C16H16N6O/c1-8(2)22-16-13(15(17)18-7-19-16)14(21-22)12-6-9-5-10(23)3-4-11(9)20-12/h3-8,20,23H,1-2H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTORC2 (unknown origin) expressed in Escherichia coli after 15 mins in presence of gamma-[32]P-ATP by high-throughput scree... |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50546186

(CHEMBL4754767)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(nc(n1)-c1cnc(N)nc1C(F)F)N1CCOCC1)O2 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residue incubated for 1 hr by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00333
BindingDB Entry DOI: 10.7270/Q2VT1WPR |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529402

(CHEMBL4517253)Show SMILES CC(C)n1nc(-c2ccc3oc(N)nc3c2)c2c1nc(C)[nH]c2=O Show InChI InChI=1S/C16H16N6O2/c1-7(2)22-14-12(15(23)19-8(3)18-14)13(21-22)9-4-5-11-10(6-9)20-16(17)24-11/h4-7H,1-3H3,(H2,17,20)(H,18,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50529396

(CHEMBL4538629)Show InChI InChI=1S/C17H19N7O/c1-3-4-7-24-15-13(9(2)20-16(18)22-15)14(23-24)10-5-6-12-11(8-10)21-17(19)25-12/h5-6,8H,3-4,7H2,1-2H3,(H2,19,21)(H2,18,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A431 cells assessed as AKT phosphorylation at S473 residue incubated for 3 hrs by HTRF assay |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50505410
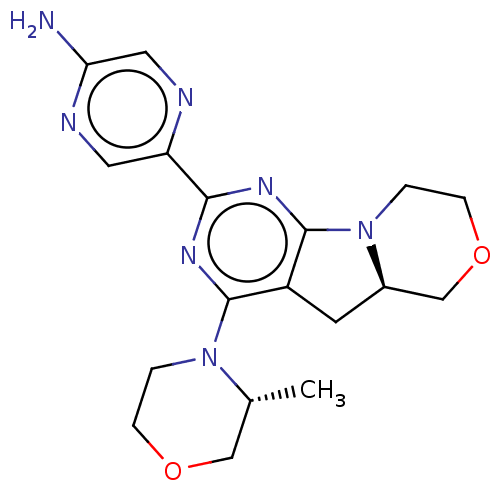
(CHEMBL4551080)Show SMILES [H][C@]12Cc3c(nc(nc3N3CCOC[C@H]3C)-c3cnc(N)cn3)N1CCOC2 |r| Show InChI InChI=1S/C18H23N7O2/c1-11-9-26-4-2-24(11)17-13-6-12-10-27-5-3-25(12)18(13)23-16(22-17)14-7-21-15(19)8-20-14/h7-8,11-12H,2-6,9-10H2,1H3,(H2,19,21)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residues incubated for 1 hr by Western blot analysis |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972
BindingDB Entry DOI: 10.7270/Q2DJ5JW1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data