

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50078827 2-(pyridin-3-yl)-1H-benzo[d]imidazole::2-Pyridin-3-yl-1H-benzoimidazole::CHEMBL83103::LDHA Inhibitor, 10
SMILES: c1ccc2[nH]c(nc2c1)-c1cccnc1
InChI Key: InChIKey=BOUOQESVDURNSB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase A (Rattus norvegicus (Rat)) | BDBM50078827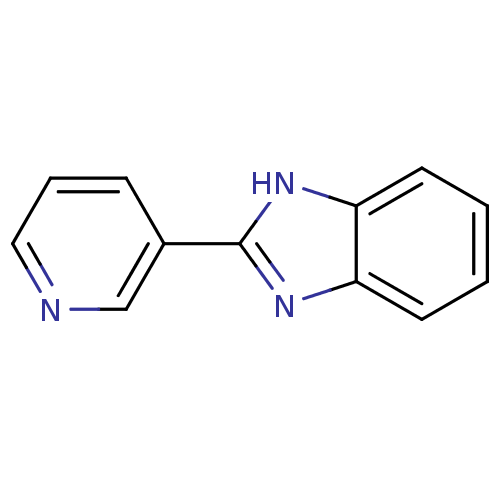 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca | Assay Description NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... | J Med Chem 55: 3285-306 (2012) Article DOI: 10.1021/jm201734r BindingDB Entry DOI: 10.7270/Q21J9896 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A (Rattus norvegicus (Rat)) | BDBM50078827 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca | Assay Description Enzyme assay using lactate dehydrogenase A (LDHA). | J Med Chem 55: 3285-306 (2012) Article DOI: 10.1021/jm201734r BindingDB Entry DOI: 10.7270/Q21J9896 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50078827 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine Aminopeptidase (MAP) (Escherichia coli (strain K12)) | BDBM50078827 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of Co2+ loaded MetAP expressed in Escherichia coli | J Med Chem 49: 511-22 (2006) Article DOI: 10.1021/jm050476z BindingDB Entry DOI: 10.7270/Q2N58KXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50078827 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50078827 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Dissociation constant when binding to FK506 binding protein (FKBP). | J Med Chem 42: 2498-503 (1999) Article DOI: 10.1021/jm990073x BindingDB Entry DOI: 10.7270/Q2639NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||