 Found 19 hits for monomerid = 50107347
Found 19 hits for monomerid = 50107347 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347
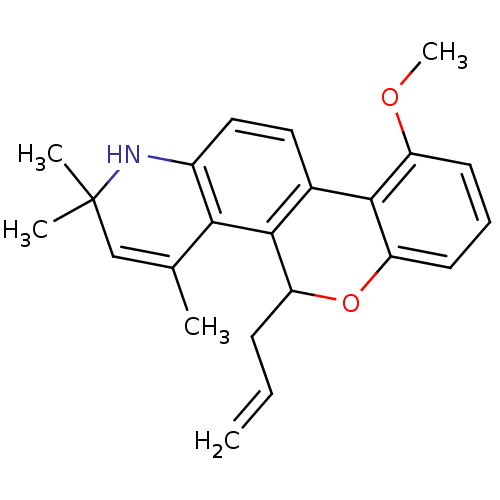
((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards glucocorticoid receptor (GR) by displacing [3H]-Dexamethasone |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase DHFR in Pneumocystis carinii. |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Androgen Receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards testosterone receptor |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-progesterone from human Progesterone receptor A |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor (PR) by displacing [3H]-progesterone. |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor (PR) by displacing [3H]-progesterone. |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase DHFR in Pneumocystis carinii. |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 780 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase DHFR in Pneumocystis carinii. |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration in HepG2 cells transfected with LUC gene (E-sel-Luc). |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid
(RAT) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of conconavalin A stimulated rat splenocyte proliferation |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing human glucocorticoid receptor |
J Med Chem 46: 1016-30 (2003)
Article DOI: 10.1021/jm020335m
BindingDB Entry DOI: 10.7270/Q2MP52NB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Rattus norvegicus) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
Forschungszentrum Dresden-Rossendorf e.V.
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 17: 4035-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.094
BindingDB Entry DOI: 10.7270/Q25X2CRN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The effective concentration in CV-1 cells for glucocorticoid response element activation (GRE). |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration in HepG2 cells transfected with LUC gene (E-sel-Luc). |
J Med Chem 44: 4481-91 (2001)
BindingDB Entry DOI: 10.7270/Q2CZ36F4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of transcriptional repression in CV-1 cells expressing glucocorticoid receptor |
Bioorg Med Chem Lett 14: 1721-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.044
BindingDB Entry DOI: 10.7270/Q2KP81M9 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of transcriptional activation in CV-1 cells expressing glucocorticoid receptor |
Bioorg Med Chem Lett 14: 1721-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.044
BindingDB Entry DOI: 10.7270/Q2KP81M9 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Forschungszentrum Dresden-Rossendorf e.V.
Curated by ChEMBL
| Assay Description
Binding affinity to rat glucocorticoid receptor |
Bioorg Med Chem Lett 17: 4035-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.094
BindingDB Entry DOI: 10.7270/Q25X2CRN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of transcriptional repression in CV-1 cells expressing glucocorticoid receptor |
Bioorg Med Chem Lett 14: 1721-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.044
BindingDB Entry DOI: 10.7270/Q2KP81M9 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50107347

((+)5-Allyl-10-methoxy-2,2,4-trimethyl-2,5-dihydro-...)Show SMILES COc1cccc2OC(CC=C)c3c(ccc4NC(C)(C)C=C(C)c34)-c12 |t:21| Show InChI InChI=1S/C23H25NO2/c1-6-8-18-22-15(21-17(25-5)9-7-10-19(21)26-18)11-12-16-20(22)14(2)13-23(3,4)24-16/h6-7,9-13,18,24H,1,8H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of transcriptional repression in CV-1 cells expressing glucocorticoid receptor |
Bioorg Med Chem Lett 14: 1721-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.044
BindingDB Entry DOI: 10.7270/Q2KP81M9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data