

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50427622 CHEMBL2323508::US9346803, Table 2, Compound 10: 3-[1-(4-chlorobenzoyl)-3-ethyl-5-methoxy-1H-indol-2-yl]propanoic acid
SMILES: CCc1c(CCC(O)=O)n(C(=O)c2ccc(Cl)cc2)c2ccc(OC)cc12
InChI Key: InChIKey=JBMSOEFHPCTJTH-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM50427622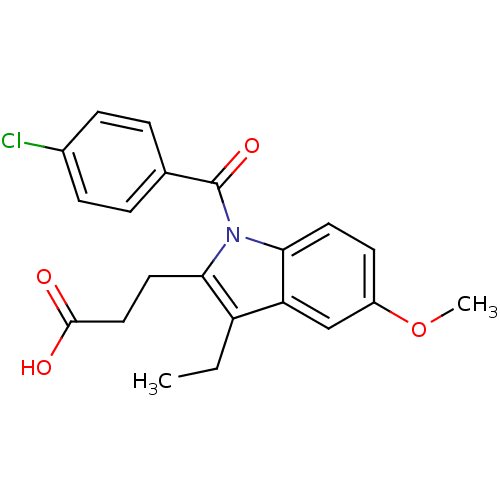 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (AK1C4) (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (AK1C4) (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphthol | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-2 (COX-2) (Mus musculus (Mouse)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of wild type mouse COX2 by discontinuous radioactive TLC assay | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||