

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM84460 HIV-1 PR Inhibitor, compound 6
SMILES: COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@@H]2[C@H](O)Cc3ccccc23)nn1
InChI Key: InChIKey=MEWAZRJLRMEJDV-FWGCVNADSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460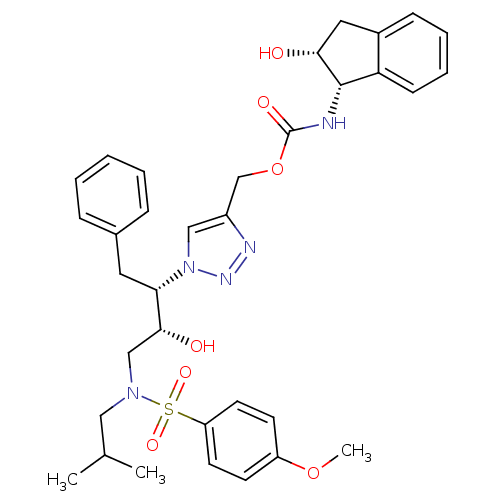 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 1.70 | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 51: 6263-70 (2008) Article DOI: 10.1021/jm800149m BindingDB Entry DOI: 10.7270/Q2MW2KZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 10 | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 22 | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 27 | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 51: 6263-70 (2008) Article DOI: 10.1021/jm800149m BindingDB Entry DOI: 10.7270/Q2MW2KZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of HIV1 protease after 24 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||