

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50030475 7-Dehydrocholesterol::CHEBI:28940::CHOLECALCIFEROL::Cholecalciferol::Colecalciferol::Dihydrocholesterol::Vitamin D::Vitamin D 3
SMILES: [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)CCC1=C)[C@H](C)CCCC(C)C
InChI Key: InChIKey=QYSXJUFSXHHAJI-YRZJJWOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50030475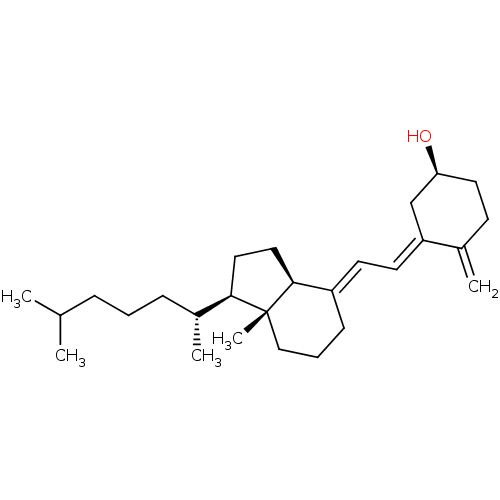 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Agonist activity at human VDR expressed in HEK293 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 5265-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.053 BindingDB Entry DOI: 10.7270/Q2S46TKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Potentiation of human GlyR-alpha1 expressed in Xenopus laevis oocytes assessed as induction of glycine-activated currents after 1 to 4 days by two-el... | J Med Chem 58: 2958-66 (2015) Article DOI: 10.1021/jm501873p BindingDB Entry DOI: 10.7270/Q22F7Q4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D receptor (Mus musculus) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Activation of VDR in mouse ASZ001 cells assessed as change in Cyp24A1 mRNA expression after 48 hrs by q-PCR analysis | Eur J Med Chem 162: 495-506 (2019) Article DOI: 10.1016/j.ejmech.2018.11.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HSP60/HSP10 (Homo sapiens) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal octa-His-tagged HSP60 expressed in Escherichia coli Rosetta(DE3) pLysS/human HSP10 expressed in Escherichia coli Roset... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 60 kDa chaperonin (Escherichia coli) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Escherichia coli GroEL expressed in Escherichia coliDH5alpha incubated for 60 mins using ATP by spectrometric analys... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 10 kDa chaperonin (Escherichia coli) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coliDH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed a... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 10 kDa chaperonin (Escherichia coli) | BDBM50030475 (7-Dehydrocholesterol | CHEBI:28940 | CHOLECALCIFER...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||