

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM2681 3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione::Bisindolyl deriv. 11::CHEMBL268769
SMILES: CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12
InChI Key: InChIKey=WHOOZDLAJIKMBZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-Dependent Protein Kinase (PKA) (Bos taurus (bovine)) | BDBM2681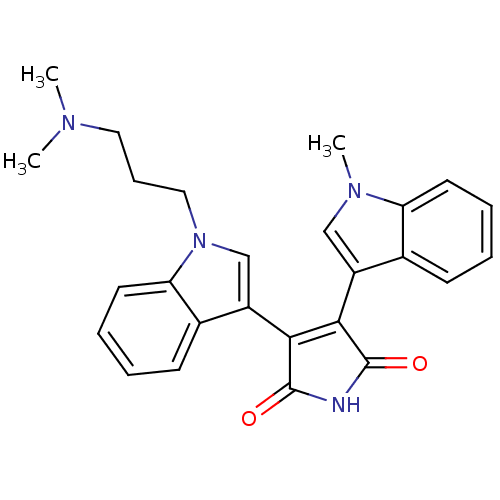 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-Dependent Protein Kinase (PKA) (Rattus norvegicus (rat)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphorylase Kinase (Oryctolagus cuniculus (rabbit)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Laboratoires Glaxo | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Biol Chem 266: 15771-81 (1991) BindingDB Entry DOI: 10.7270/Q2FF3QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Kinase C (Rattus norvegicus (rat)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 994-1001 (1992) Article DOI: 10.1021/jm00084a004 BindingDB Entry DOI: 10.7270/Q2M043KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant Pim1 expressed in insect cells by HTRF | Bioorg Med Chem Lett 19: 1512-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.005 BindingDB Entry DOI: 10.7270/Q2RF5VX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-chymotrypsin (Homo sapiens (Human)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-Dependent Protein Kinase (PKA) (Bos taurus (bovine)) | BDBM2681 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone. | J Med Chem 35: 994-1001 (1992) Article DOI: 10.1021/jm00084a004 BindingDB Entry DOI: 10.7270/Q2M043KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||