

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10338 (S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-indole-2,3-dione::5-{[(2S)-2-(phenoxymethyl)azetidine-1-]sulfonyl}-2,3-dihydro-1H-indole-2,3-dione::Azetidine Isatin Analogue 17
SMILES: O=C1Nc2ccc(cc2C1=O)S(=O)(=O)N1CC[C@H]1COc1ccccc1
InChI Key: InChIKey=WWKKPLWKYQGHBF-LBPRGKRZSA-N
PDB links: 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-3 (Homo sapiens (Human)) | BDBM10338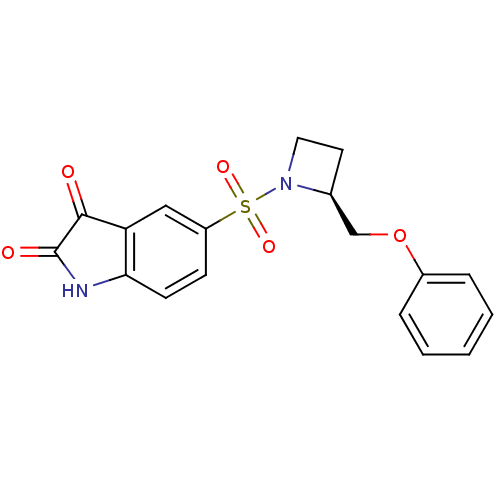 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 287 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Washington University School of Medicine | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 7637-47 (2005) Article DOI: 10.1021/jm0506625 BindingDB Entry DOI: 10.7270/Q2V40SDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 7637-47 (2005) Article DOI: 10.1021/jm0506625 BindingDB Entry DOI: 10.7270/Q2V40SDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 7637-47 (2005) Article DOI: 10.1021/jm0506625 BindingDB Entry DOI: 10.7270/Q2V40SDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of caspase3 | Bioorg Med Chem 20: 5410-5 (2012) Article DOI: 10.1016/j.bmc.2012.03.041 BindingDB Entry DOI: 10.7270/Q2GF0VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 7637-47 (2005) Article DOI: 10.1021/jm0506625 BindingDB Entry DOI: 10.7270/Q2V40SDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.57E+3 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of staurosporine induced activation of caspase 3 activation in human Hela cells assessed as hydrolysis of Z-DEVD-R110 substrate by micropl... | Bioorg Med Chem Lett 21: 2192-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.015 BindingDB Entry DOI: 10.7270/Q2TB176G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10338 ((S)-5-(2-Phenoxymethyl-azetidine-1-sulfonyl)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors. The amount of AMC released was determined by using a ... | J Med Chem 48: 7637-47 (2005) Article DOI: 10.1021/jm0506625 BindingDB Entry DOI: 10.7270/Q2V40SDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||