

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13242 2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide::2-[[(Butylamino)-carbonyl]amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide::BMS-354825 2-Amino-4-methyl-thiazole Analog 8c::CHEMBL131346
SMILES: CCCCNC(=O)Nc1nc(C)c(s1)C(=O)Nc1c(C)cc(C)cc1C
InChI Key: InChIKey=ANOOKLPIDKBMTR-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13242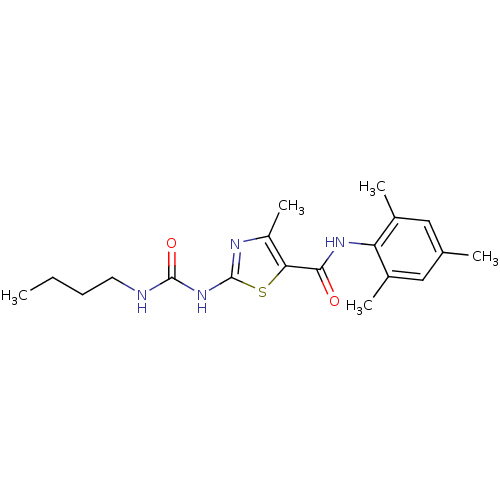 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13242 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13242 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13242 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||