

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM14323 Fragment 20::quinazoline-2,4-diamine
SMILES: Nc1nc(N)c2ccccc2n1
InChI Key: InChIKey=XELRMPRLCPFTBH-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer. 12 PDB IDs contain this monomer as substructures. 12 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14323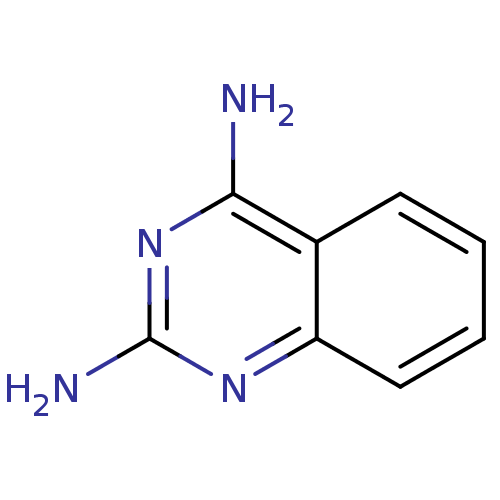 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14323 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM14323 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase of rat liver | J Med Chem 24: 812-8 (1981) BindingDB Entry DOI: 10.7270/Q2P84D3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM14323 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Concentration required for the inhibition of acetylcholinesterase | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||