

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM152706 Chlorhexidine::Chlorhexidine (1)
SMILES: Clc1ccc(NC(=N)NC(=N)NCCCCCCNC(=N)NC(=N)Nc2ccc(Cl)cc2)cc1
InChI Key: InChIKey=GHXZTYHSJHQHIJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylpolyamine oxidase (APAO) (Homo sapiens (Human)) | BDBM152706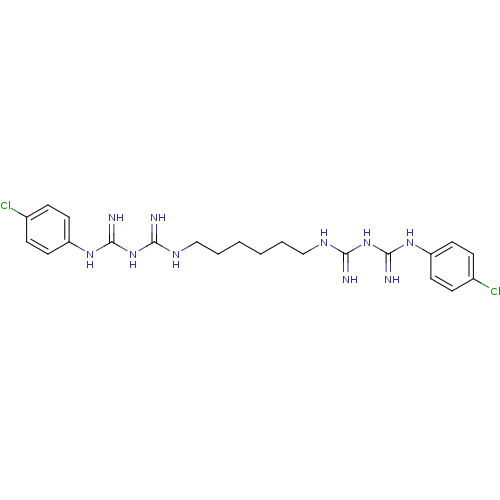 (Chlorhexidine | Chlorhexidine (1)) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 100 | -9.54 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University Roma Tre | Assay Description The APAO and SMO activities were measured spectrophotometrically by following the formation of a pink adduct (ε515 nm = 2.6 × 10^4 M−1 cm&... | J Enzyme Inhib Med Chem 28: 463-7 (2013) Article DOI: 10.3109/14756366.2011.650691 BindingDB Entry DOI: 10.7270/Q2X065ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermine oxidase (SMO) (Homo sapiens (Human)) | BDBM152706 (Chlorhexidine | Chlorhexidine (1)) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 550 | -8.53 | n/a | n/a | n/a | n/a | n/a | 8.3 | 25 |
University Roma Tre | Assay Description The APAO and SMO activities were measured spectrophotometrically by following the formation of a pink adduct (ε515 nm = 2.6 × 10^4 M−1 cm&... | J Enzyme Inhib Med Chem 28: 463-7 (2013) Article DOI: 10.3109/14756366.2011.650691 BindingDB Entry DOI: 10.7270/Q2X065ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eis_Ban acetyltransferase (Eis_Ban) (Bacillus anthracis str. Sterne) | BDBM152706 (Chlorhexidine | Chlorhexidine (1)) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Kentucky | Assay Description Briefly, reactions (200 μL) contained compounds 1−3 dissolved in Tris-HCl buffer (50 mM, pH 8.0, 10% DMSO) with a 5-fold serial dilution, ... | Biochemistry 54: 3197-206 (2015) Article DOI: 10.1021/acs.biochem.5b00244 BindingDB Entry DOI: 10.7270/Q2TT4PP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||