

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM167912 N,N-diethylcarbamic acid-4-(5-hydroxy-6,7-dimethoxy-4-oxo-4H-1-benzopyran-2-yl)phenyl ester (7j)
SMILES: CCN(CC)C(=O)Oc1ccc(cc1)-c1cc(=O)c2c(O)c(OC)c(OC)cc2o1
InChI Key: InChIKey=BCPMGKFDBYFFIG-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM167912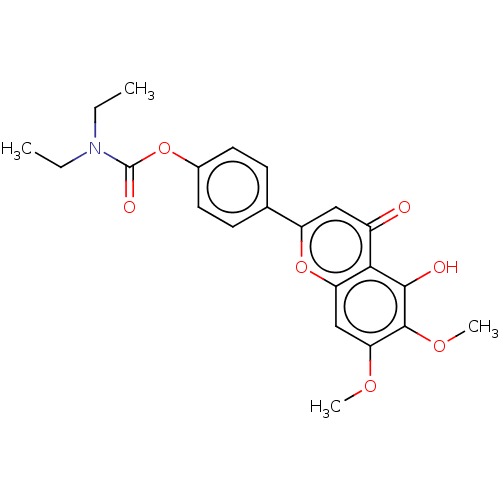 (N,N-diethylcarbamic acid-4-(5-hydroxy-6,7-dimethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description For rat AChE or BuChE inhibition assays, a reaction mixture (100 μL) containing acetylthiocholine iodide (1 mmol/L, 30 μL) (J&K Scientific)... | Chem Biol Drug Des 86: 1168-77 (2015) Article DOI: 10.1111/cbdd.12580 BindingDB Entry DOI: 10.7270/Q2VM4B1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM167912 (N,N-diethylcarbamic acid-4-(5-hydroxy-6,7-dimethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 668-80 (2015) Article DOI: 10.1016/j.bmc.2015.01.005 BindingDB Entry DOI: 10.7270/Q24T6M2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM167912 (N,N-diethylcarbamic acid-4-(5-hydroxy-6,7-dimethox...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description For rat AChE or BuChE inhibition assays, a reaction mixture (100 μL) containing acetylthiocholine iodide (1 mmol/L, 30 μL) (J&K Scientific)... | Chem Biol Drug Des 86: 1168-77 (2015) Article DOI: 10.1111/cbdd.12580 BindingDB Entry DOI: 10.7270/Q2VM4B1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||