

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18363 (2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidine-3,4,5-triol::CO-DNJ::alpha-1-C-Octyl-DNJ
SMILES: CCCCCCCC[C@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O
InChI Key: InChIKey=IIHXPVAHXKBRBL-RKQHYHRCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18363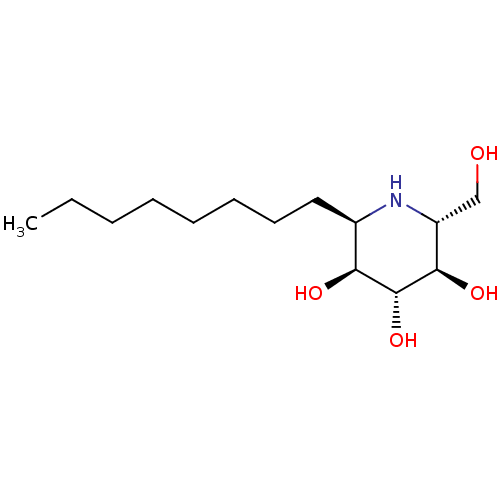 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -9.29 | 500 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic alpha-glucosidase (Rattus norvegicus) | BDBM18363 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM18363 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Glucosidase (alpha-Glu) (Homo sapiens (Human)) | BDBM18363 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM18363 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||