

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18506 6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,9,12,14-heptaen-2-ylmethyl}pteridine-2,4-diamine::CHEMBL301769::Pteridine compound, 17
SMILES: Nc1nc(N)c2nc(CN3c4ccccc4C=Cc4ccccc34)cnc2n1
InChI Key: InChIKey=NXCCIJQEAKMFGW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate Reductase (DHFR) (Bacillus cereus (ATCC 14579)) | BDBM18506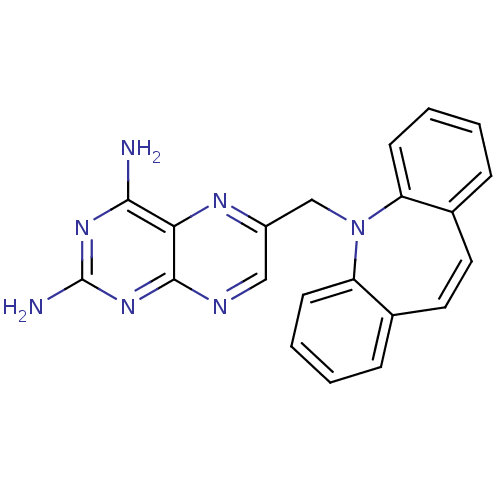 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the rate of enzyme-dependent NADPH consumption at an absorbance of 340 nm over a period of severa... | Antimicrob Agents Chemother 50: 3435-43 (2006) Article DOI: 10.1128/AAC.00386-06 BindingDB Entry DOI: 10.7270/Q2FQ9TVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Mycobacterium avium | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Rat liver | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate Reductase (DHFR) (Pneumocystis carinii) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Pneumocystis carinii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Toxoplasma gondii) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Toxoplasma gondii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Concentration required to inhibit the Pneumocystis carinii Dihydrofolate reductase by 50% was determined; Range : 58-110 | J Med Chem 47: 2475-85 (2004) Article DOI: 10.1021/jm030599o BindingDB Entry DOI: 10.7270/Q2K64HHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (DHFR) (Toxoplasma gondii) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrohydrofolate reductase(DHFR) of Toxoplasma gondii | J Med Chem 46: 1726-36 (2003) Article DOI: 10.1021/jm020466n BindingDB Entry DOI: 10.7270/Q2CR5SRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Concentration required to inhibit the rat liver Dihydrofolate reductase by 50% was determined; Range : 2500-3600 | J Med Chem 47: 2475-85 (2004) Article DOI: 10.1021/jm030599o BindingDB Entry DOI: 10.7270/Q2K64HHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Concentration required to inhibit the Toxoplasma gondii Dihydrofolate reductase by 50% was determined; Range : 32-47 | J Med Chem 47: 2475-85 (2004) Article DOI: 10.1021/jm030599o BindingDB Entry DOI: 10.7270/Q2K64HHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Concentration required to inhibit the Mycobacterium avium Dihydrofolate reductase by 50% was determined; Range : 9.1-17 | J Med Chem 47: 2475-85 (2004) Article DOI: 10.1021/jm030599o BindingDB Entry DOI: 10.7270/Q2K64HHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Pneumocystis carinii (Pc) dihydrofolate reductase. | J Med Chem 45: 233-41 (2001) BindingDB Entry DOI: 10.7270/Q2SX6CJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||