

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18808 2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-4-one::CHEMBL400485::Pyrrolo[2,3-d]pyrimidine analogue, 3
SMILES: Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1
InChI Key: InChIKey=WWENIERBTIAOJG-UHFFFAOYSA-N
Data: 15 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate Synthase (TS) (Escherichia coli) | BDBM18808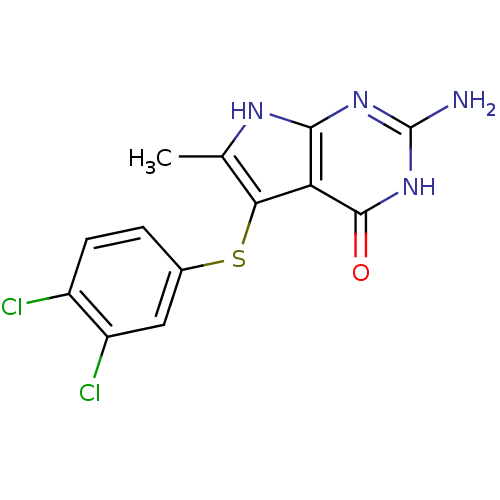 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 51: 68-76 (2008) Article DOI: 10.1021/jm701052u BindingDB Entry DOI: 10.7270/Q2057D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human thymidylate synthase | J Med Chem 51: 5789-97 (2008) Article DOI: 10.1021/jm8006933 BindingDB Entry DOI: 10.7270/Q2FF3T7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 49: 1055-65 (2006) Article DOI: 10.1021/jm058276a BindingDB Entry DOI: 10.7270/Q2833Q90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 51: 68-76 (2008) Article DOI: 10.1021/jm701052u BindingDB Entry DOI: 10.7270/Q2057D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli thymidylate synthase | J Med Chem 51: 5789-97 (2008) Article DOI: 10.1021/jm8006933 BindingDB Entry DOI: 10.7270/Q2FF3T7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthase | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound was evaluated on Streptococcus faecium Thymidylate synthase | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound against Dihydrofolate reductases on Pneumocystis carini | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound against Dihydrofolate reductases on rat liver | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound was evaluated on Lactobacillus casei Thymidylate synthase | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound was evaluated on Human Thymidylate synthase | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound was evaluated on Escherichia coli Thymidylate synthase | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Toxoplasma gondii) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description The inhibitory concentration of compound against Dihydrofolate reductases on Toxoplasma gondii | J Med Chem 39: 4563-8 (1996) Article DOI: 10.1021/jm960097t BindingDB Entry DOI: 10.7270/Q27W6CVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration against human thymidylate synthase | Bioorg Med Chem Lett 15: 2225-30 (2005) Article DOI: 10.1016/j.bmcl.2005.03.029 BindingDB Entry DOI: 10.7270/Q2ZW1MQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18808 (2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 51: 68-76 (2008) Article DOI: 10.1021/jm701052u BindingDB Entry DOI: 10.7270/Q2057D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||