

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM198 (2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-methyl-2-{[methyl(pyridin-2-ylmethyl)carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]-3-methyl-2-{[methyl(pyridin-2-ylmethyl)carbamoyl]amino}butanamide::A-76928::JMC51852 Inhibitor PP
SMILES: CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C
InChI Key: InChIKey=QPVWMQXBTCSLCB-UNHORJANSA-N
PDB links: 2 PDB IDs match this monomer. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM198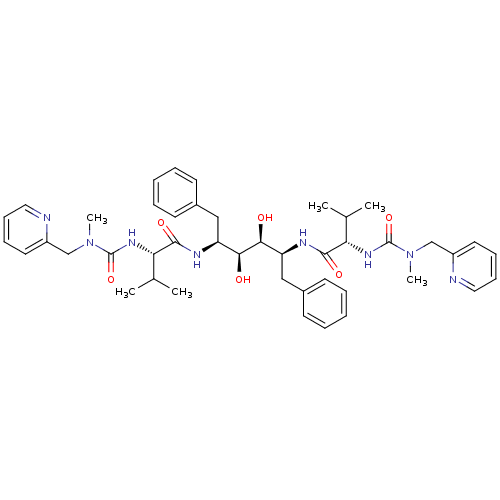 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article | 0.0110 | -15.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Am Chem Soc 116: 847-55 (1994) Article DOI: 10.1021/ja00082a004 BindingDB Entry DOI: 10.7270/Q2KK98Z1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM198 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.30 | -12.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Universidad de Santiago de Compostela | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | J Med Chem 51: 852-60 (2008) Article DOI: 10.1021/jm701170f BindingDB Entry DOI: 10.7270/Q2QN652X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 Protease Mutant (V82A) (Human immunodeficiency virus type 1) | BDBM198 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9 | -11.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Universidad de Santiago de Compostela | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | J Med Chem 51: 852-60 (2008) Article DOI: 10.1021/jm701170f BindingDB Entry DOI: 10.7270/Q2QN652X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM198 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 0.0759 | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Affinity for HIV Protease | J Med Chem 45: 2770-80 (2002) Article DOI: 10.1021/jm0105833 BindingDB Entry DOI: 10.7270/Q2MG7S8S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM198 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity | Eur J Med Chem 44: 4344-52 (2009) Article DOI: 10.1016/j.ejmech.2009.05.016 BindingDB Entry DOI: 10.7270/Q2JH3Q0K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||