

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O
InChI Key: InChIKey=ZHUJMSMQIPIPTF-IBURTVSXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21025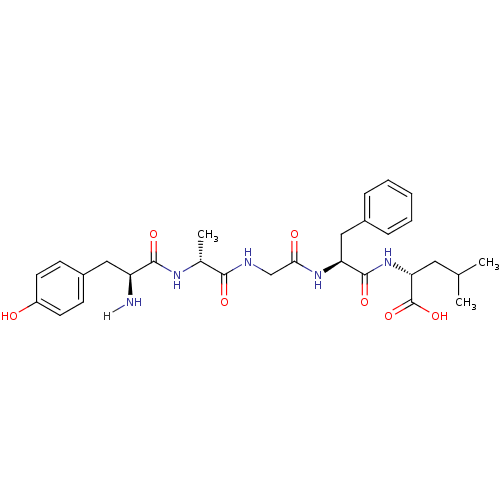 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [3H]-U-69,593 radioligand binding to Guinea pig Opioid receptor kappa 1; not determined | J Med Chem 38: 1523-37 (1995) BindingDB Entry DOI: 10.7270/Q2MG7Q5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Opioid receptor kappa 1 affinity against the receptor site model site 4(kappa) | J Med Chem 29: 531-7 (1986) BindingDB Entry DOI: 10.7270/Q2WH2QKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against the Opioid receptor delta 1 | J Med Chem 29: 531-7 (1986) BindingDB Entry DOI: 10.7270/Q2WH2QKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Opioid receptor mu 2 affinity against the receptor site model site 2(mu2) | J Med Chem 29: 531-7 (1986) BindingDB Entry DOI: 10.7270/Q2WH2QKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency against Opioid receptor mu 1 in longitudinal muscle preparation of guinea pig ileum | J Med Chem 24: 1119-24 (1982) BindingDB Entry DOI: 10.7270/Q2QC0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human DOR expressed in CHOK1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation incubated for 30 mins by lumi... | Citation and Details Article DOI: 10.1039/d0md00104j BindingDB Entry DOI: 10.7270/Q2K64NQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21025 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency against delta Opioid receptor delta 1 in longitudinal muscle preparation of mouse vas deferens | J Med Chem 24: 1119-24 (1982) BindingDB Entry DOI: 10.7270/Q2QC0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| << First | Previous | Displayed 51 to 57 (of 57 total ) |