

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM214689 US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol
SMILES: Cc1cc(Nc2nc(nc3ccccc23)[C@@H](O)c2ccc(F)cc2)n[nH]1
InChI Key: InChIKey=DCRWIATZWHLIPN-KRWDZBQOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM214689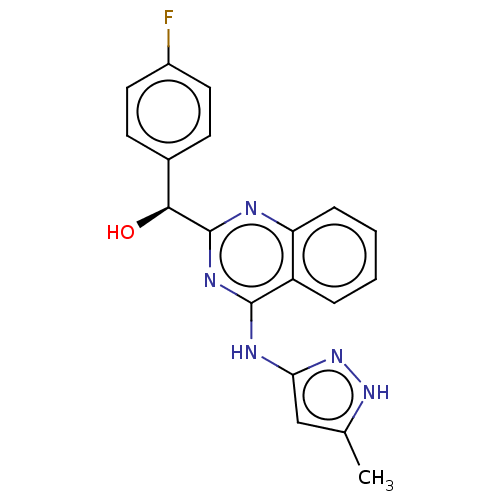 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 25 |
Ambit Biosciences Corporation US Patent | Assay Description For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
Arromax Pharmatech Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) assessed as dissociate constant | Eur J Med Chem 170: 55-72 (2019) Article DOI: 10.1016/j.ejmech.2019.03.004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 25 |
Ambit Biosciences Corporation US Patent | Assay Description For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 25 |
Ambit Biosciences Corporation US Patent | Assay Description For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.42 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <310 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Ambit Biosciences Corporation US Patent | Assay Description The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
Arromax Pharmatech Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) assessed as dissociate constant | Eur J Med Chem 170: 55-72 (2019) Article DOI: 10.1016/j.ejmech.2019.03.004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Arromax Pharmatech Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) assessed as dissociate constant | Eur J Med Chem 170: 55-72 (2019) Article DOI: 10.1016/j.ejmech.2019.03.004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 25 |
Ambit Biosciences Corporation US Patent | Assay Description For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||