

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM227643 Vinblastine
SMILES: CC[C@]1(O)C[C@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC
InChI Key: InChIKey=JXLYSJRDGCGARV-XQKSVPLYSA-N
Data: 13 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-gp nanodisc·BD-verapamil (Mus musculus (Mouse)) | BDBM227643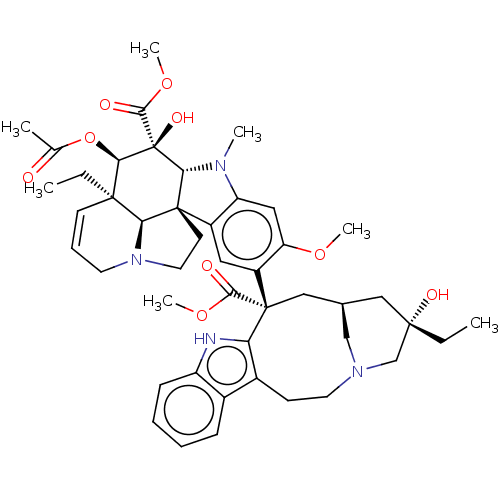 (Vinblastine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | 22.5 |
University of Washington | Assay Description For the data shown, 25 nM BD-verapamil was added to various P-gp nanodisc concentrations in the presence of 1 μM empty nanodiscs, and 50 nM BD-v... | Biochemistry 56: 2506-2517 (2017) Article DOI: 10.1021/acs.biochem.6b01245 BindingDB Entry DOI: 10.7270/Q20G3J1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-gp nanodisc·BD-vinblastine (Mus musculus (Mouse)) | BDBM227643 (Vinblastine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | 22.5 |
University of Washington | Assay Description For the data shown, 25 nM BD-verapamil was added to various P-gp nanodisc concentrations in the presence of 1 μM empty nanodiscs, and 50 nM BD-v... | Biochemistry 56: 2506-2517 (2017) Article DOI: 10.1021/acs.biochem.6b01245 BindingDB Entry DOI: 10.7270/Q20G3J1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t... | Toxicol Sci 118: 485-500 (2010) Article DOI: 10.1093/toxsci/kfq269 BindingDB Entry DOI: 10.7270/Q26Q20JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1/beta type-5 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 26S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome Macropain subunit (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 26S proteasome using Boc-LRR-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome component C5 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 26S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome component C5 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome Macropain subunit (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome using Boc-LRR-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1/beta type-5 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM227643 (Vinblastine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||