

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22864 2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1-amine::Histaprodifen
SMILES: NCCc1cnc(CCC(c2ccccc2)c2ccccc2)[nH]1
InChI Key: InChIKey=BPZGZNLONLANFE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22864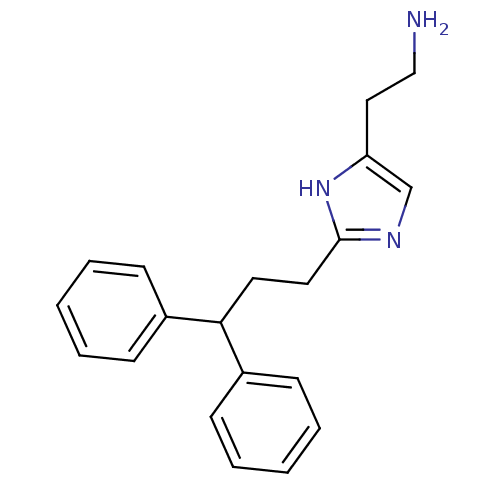 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.776 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Antagonism of guinea pig ileum contraction at 100 nM mepyramine | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTR4 (RAT) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards 5-hydroxytryptamine 4 receptor of rat | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 3a (5-HT3a)/3b (5-HT3b) receptor (Homo sapiens (Human)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards 5-hydroxytryptamine 3 receptor of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for mepyramine antagonism effects in rat aorta (relaxation) at a mepyramine concentration of 100 nM | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards 5-hydroxytryptamine 2A receptor of rat | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards 5-hydroxytryptamine 1B receptor of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.977 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for mepyramine antagonism effects in guinea pig aorta (contraction) at a mepyramine concentration of 100 nM | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards Alpha-1D adrenergic receptor of rat | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Cavia porcellus) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards Muscarinic acetylcholine receptor M3 of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards Histamine H2 receptor of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (GUINEA PIG) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | >1.58E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards Histamine H3 receptor of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for mepyramine antagonism effects in guinea pig aorta (contraction) at a mepyramine concentration of 100 nM | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (GUINEA PIG) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 4.57E+4 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was evaluated for its antagonist affinity towards Beta-1 adrenergic receptor of guinea pig | J Med Chem 43: 1071-84 (2000) BindingDB Entry DOI: 10.7270/Q2MC918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Maximum response (E max) against Histamine H1 receptor in rat aorta | J Med Chem 46: 5458-70 (2003) Article DOI: 10.1021/jm0309147 BindingDB Entry DOI: 10.7270/Q2CN753N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22864 (2-[2-(3,3-diphenylpropyl)-1H-imidazol-4-yl]ethan-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibitory activity against human Histamine H1 receptor | J Med Chem 46: 5812-24 (2003) Article DOI: 10.1021/jm030936t BindingDB Entry DOI: 10.7270/Q2GT5PF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||