

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22947 1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-1H-indol-3-yl}-1-hydroxybutan-2-yl]-1H-imidazole-4-carboxamide::CHEMBL340297::Hybrid compound 1(FR235999) derivative, 8c
SMILES: Cn1cc(CC[C@H](CO)n2cnc(c2)C(N)=O)c2cc(OCCCc3ccc(Cl)cc3)ccc12
InChI Key: InChIKey=LOLFJCSELDRAPA-OAQYLSRUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22947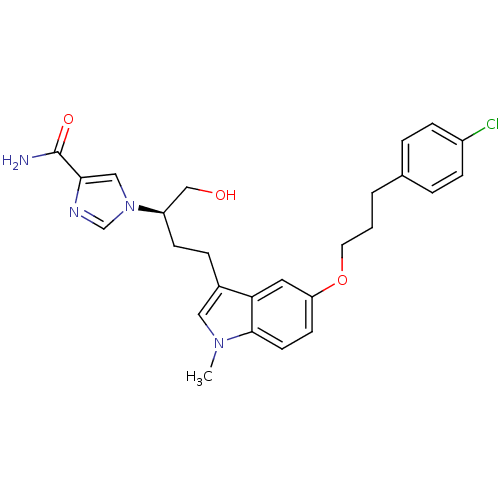 (1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -11.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22947 (1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against adenosine deaminase | J Med Chem 48: 4750-3 (2005) Article DOI: 10.1021/jm050413g BindingDB Entry DOI: 10.7270/Q2JD4WBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||