

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23197 (1S,2R,4aS,6aS,6bR,10S,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid::Ursolic Acid, 10::Ursolic acid (UA)::pentacyclic triterpene compound 10
SMILES: [H][C@@]12[C@@H](C)[C@H](C)CC[C@@]1(CC[C@]1(C)C2=CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]12C)C(O)=O
InChI Key: InChIKey=WCGUUGGRBIKTOS-JJWDWEPMSA-N
Data: 2 IC50
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM23197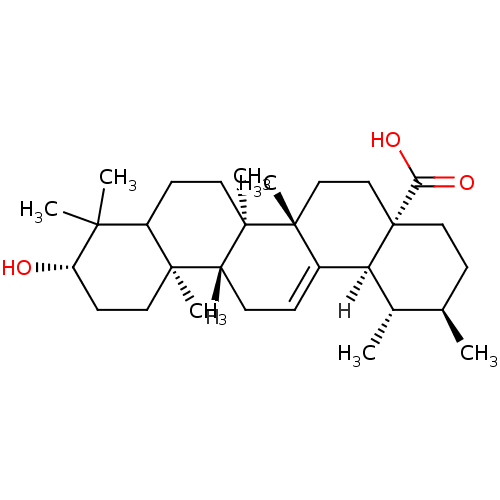 ((1S,2R,4aS,6aS,6bR,10S,12aR,12bR,14bS)-10-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 6.5 | 30 |
Yanbian University College of Pharmacy | Assay Description Briefly, the enzymatic activity of the PTP1B catalytic domain was determinedat 30°C by monitoring the hydrolysis of pNPP. Dephosphorylation of pNPP g... | J Enzyme Inhib Med Chem 28: 1199-204 (2013) Article DOI: 10.3109/14756366.2012.723206 BindingDB Entry DOI: 10.7270/Q2668C3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM23197 ((1S,2R,4aS,6aS,6bR,10S,12aR,12bR,14bS)-10-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
China Pharmaceutical University | Assay Description The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio... | J Med Chem 51: 3540-54 (2008) Article DOI: 10.1021/jm8000949 BindingDB Entry DOI: 10.7270/Q2WQ0233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||