

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23584 (3-Phenoxybenzoylamino)benzoic acid deriv., 24c::2-[(3-phenoxy-4-phenylbenzene)amido]benzoic acid
SMILES: OC(=O)c1ccccc1NC(=O)c1ccc(c(Oc2ccccc2)c1)-c1ccccc1
InChI Key: InChIKey=RYMAXBJFTSZAJG-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta-Ketoacyl-ACP Synthase III (FabH) (Enterococcus faecalis) | BDBM23584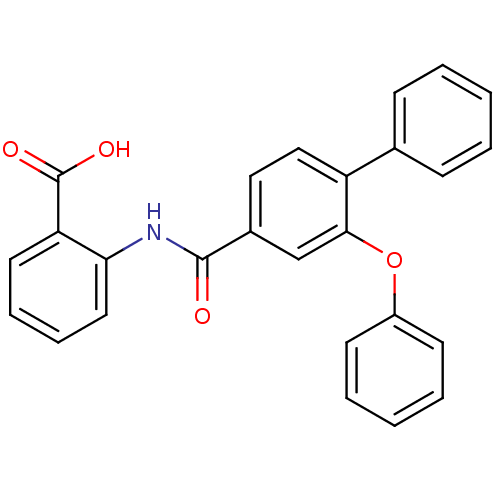 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Quorex Pharmaceuticals Inc. | Assay Description The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of a... | J Med Chem 48: 1596-609 (2005) Article DOI: 10.1021/jm049141s BindingDB Entry DOI: 10.7270/Q22B8W9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Ketoacyl-ACP Synthase III (FabH) (Streptococcus pyogenes) | BDBM23584 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Quorex Pharmaceuticals Inc. | Assay Description The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of a... | J Med Chem 48: 1596-609 (2005) Article DOI: 10.1021/jm049141s BindingDB Entry DOI: 10.7270/Q22B8W9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-heptyl-4(1H)-quinolone synthase PqsD (Pseudomonas aeruginosa) | BDBM23584 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa PqsD | J Med Chem 61: 10385-10402 (2018) Article DOI: 10.1021/acs.jmedchem.8b00540 BindingDB Entry DOI: 10.7270/Q2XP77NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Ketoacyl-ACP Synthase III (FabH) (Haemophilus influenzae) | BDBM23584 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Quorex Pharmaceuticals Inc. | Assay Description The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of a... | J Med Chem 48: 1596-609 (2005) Article DOI: 10.1021/jm049141s BindingDB Entry DOI: 10.7270/Q22B8W9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Ketoacyl-ACP Synthase III (FabH) (Enterococcus faecalis) | BDBM23584 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Shri G.S. Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis FabH by FabD/FabH coupled assay | Eur J Med Chem 43: 1071-80 (2008) Article DOI: 10.1016/j.ejmech.2007.06.018 BindingDB Entry DOI: 10.7270/Q2GQ700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Ketoacyl-ACP Synthase III (FabH) (Staphylococcus aureus) | BDBM23584 ((3-Phenoxybenzoylamino)benzoic acid deriv., 24c | ...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Quorex Pharmaceuticals Inc. | Assay Description The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of a... | J Med Chem 48: 1596-609 (2005) Article DOI: 10.1021/jm049141s BindingDB Entry DOI: 10.7270/Q22B8W9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||