

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM24692 2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5-yl)butyl]phenyl}formamido)pentanedioic acid::DDACTHF
SMILES: Nc1nc(N)c(CCCCc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(=O)[nH]1
InChI Key: InChIKey=LNUHUIPUTGDPDG-UHFFFAOYSA-N
Data: 4 KI
PDB links: 5 PDB IDs contain this monomer as substructures. 5 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycinamide ribonucleotide formyltransferase (GARFTase) (Homo sapiens (Human)) | BDBM24692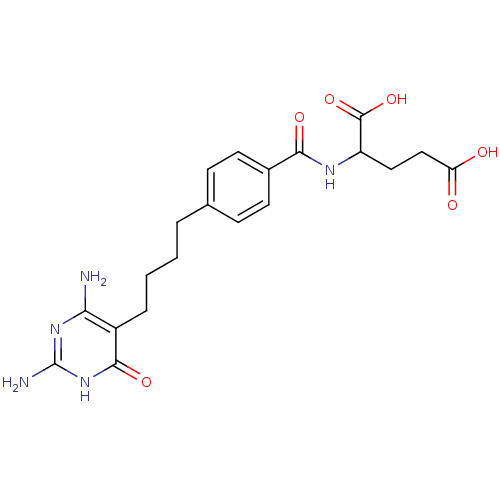 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -7.89 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycinamide ribonucleotide formyltransferase (GARFTase) (Homo sapiens (Human)) | BDBM24692 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AICAR transformylase (Homo sapiens (Human)) | BDBM24692 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AICAR Tfase (Homo sapiens (Human)) | BDBM24692 (2-({4-[4-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -6.43 | n/a | n/a | n/a | n/a | n/a | 7.4 | 26 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||