

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280415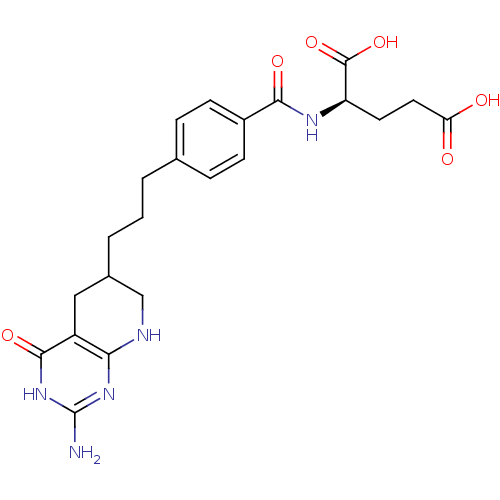 ((R)-2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.0000190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590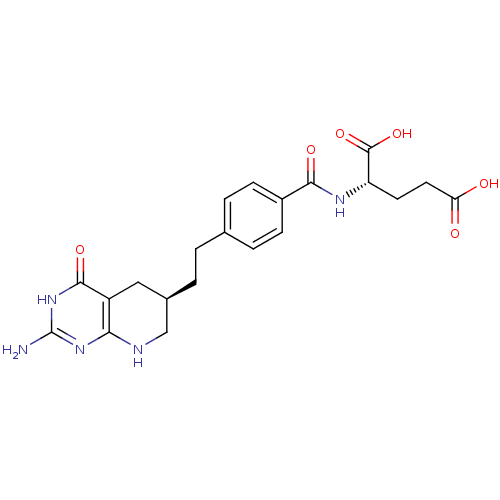 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 0.000120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280414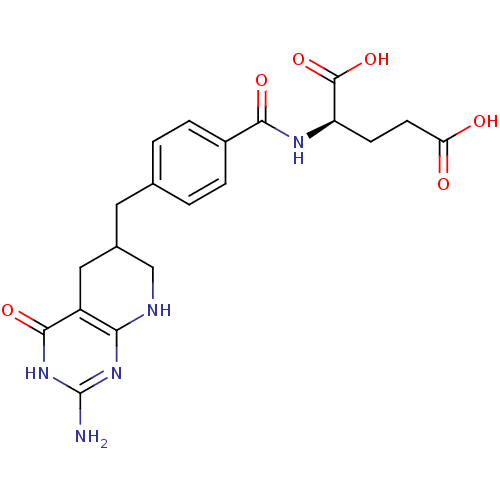 ((R)-2-[4-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059331 ((R)-2-({5-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059333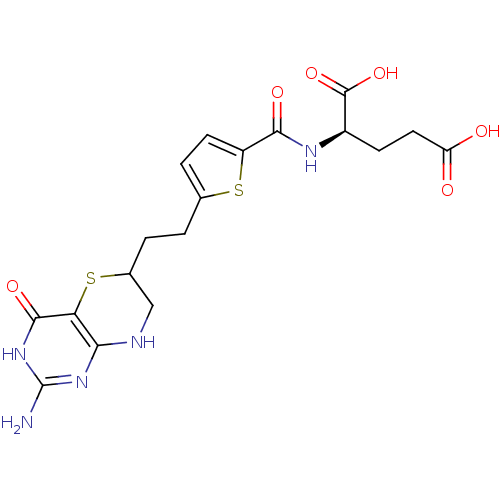 ((R)-2-({5-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059332 (2-{3-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-pyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University Curated by ChEMBL | Assay Description Inhibition of GARFTase (unknown origin) | Eur J Med Chem 115: 245-56 (2016) Article DOI: 10.1016/j.ejmech.2016.03.032 BindingDB Entry DOI: 10.7270/Q23J3H0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059342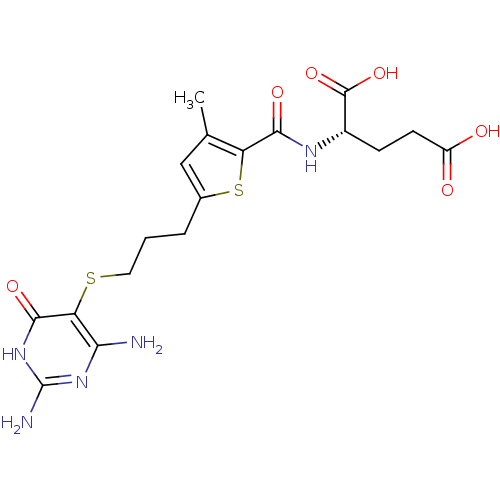 ((S)-2-({5-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50126174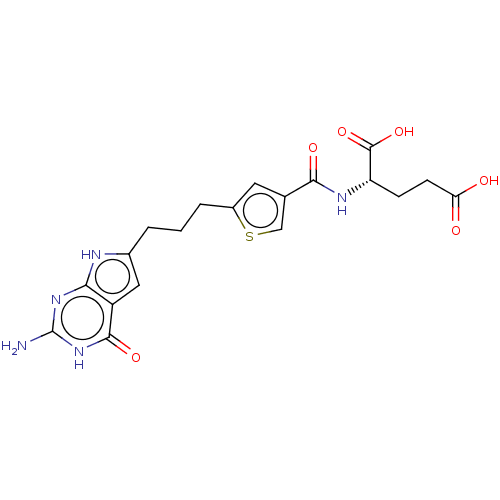 (CHEMBL3628346) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,80-dideazafolic acid measured every 1... | J Med Chem 58: 6938-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00801 BindingDB Entry DOI: 10.7270/Q2QJ7K3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50126172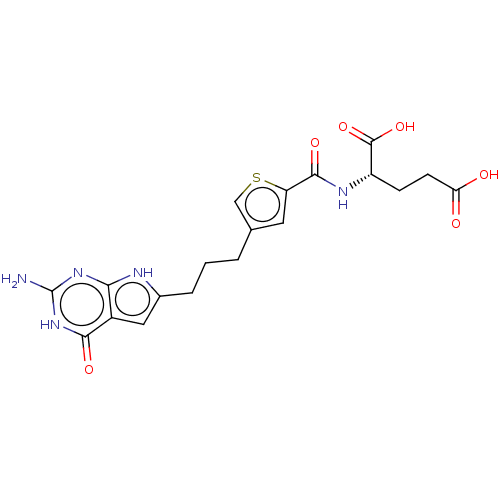 (CHEMBL3628344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,80-dideazafolic acid measured every 1... | J Med Chem 58: 6938-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00801 BindingDB Entry DOI: 10.7270/Q2QJ7K3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059337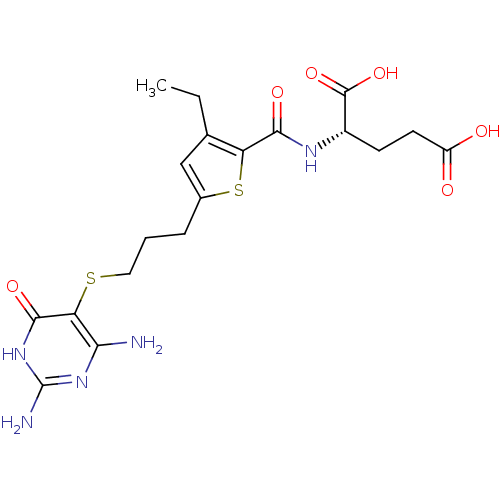 ((S)-2-({5-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059330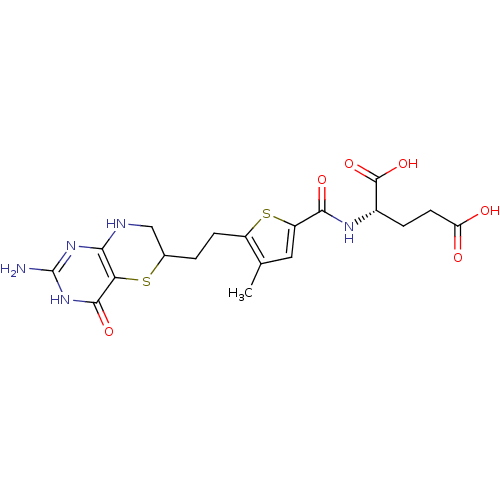 ((S)-2-({5-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059335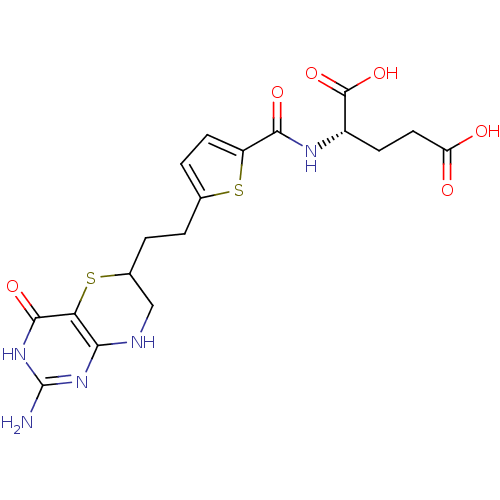 ((S)-2-({5-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24693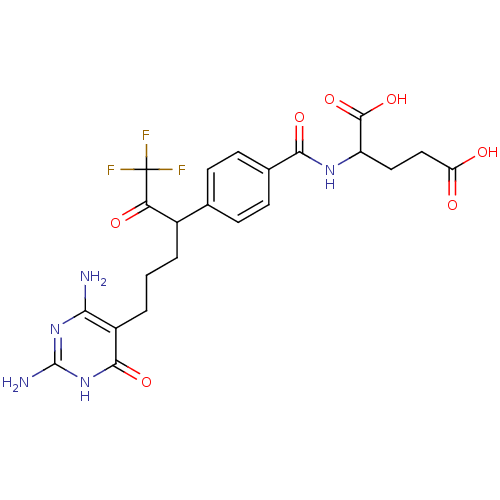 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant GAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24693 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059338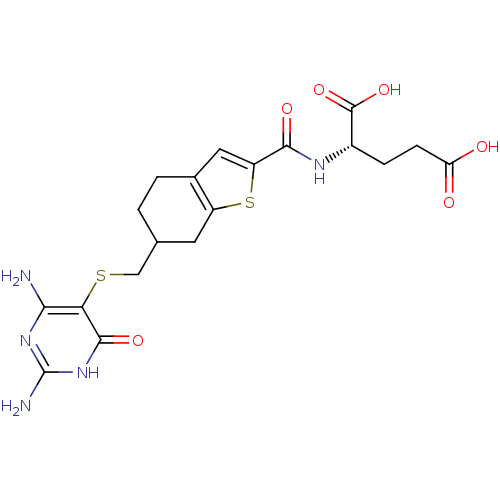 ((S)-2-{[6-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24693 (10-CF3CO-DDACTHF (5) | 10-CF3CO-DDACTHF, 2 | 2-({4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059341 ((S)-2-({5-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059334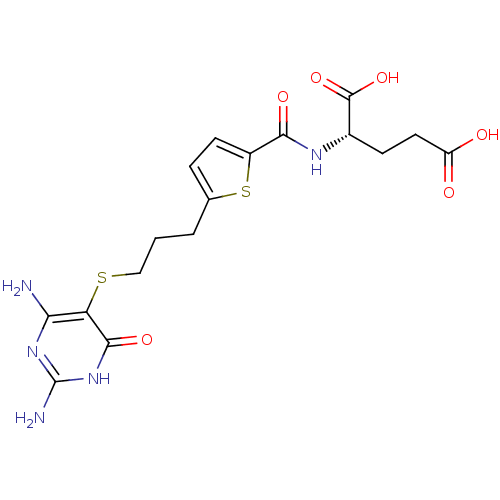 ((S)-2-({5-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059339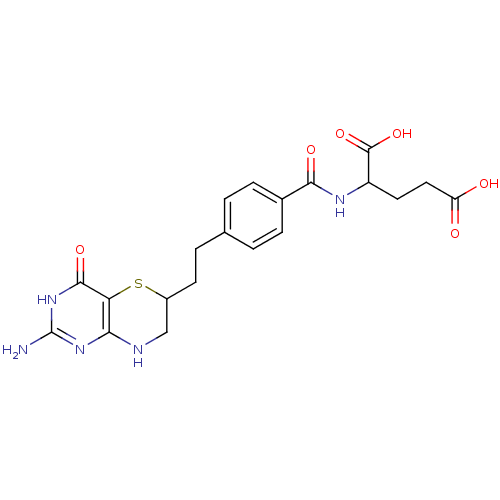 (2-{4-[2-(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-pyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50059340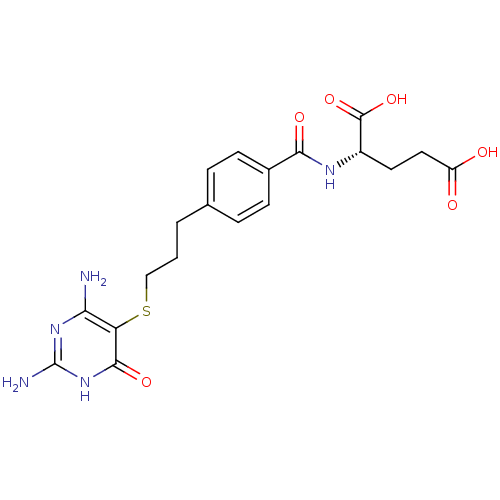 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Glycinamide Ribonucleotide Transformylase (GART) using N10-formyl-5,8-dideazafolate (FDDF) as the cofactor . | J Med Chem 40: 2502-24 (1997) Article DOI: 10.1021/jm9607459 BindingDB Entry DOI: 10.7270/Q29C6WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005868 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534427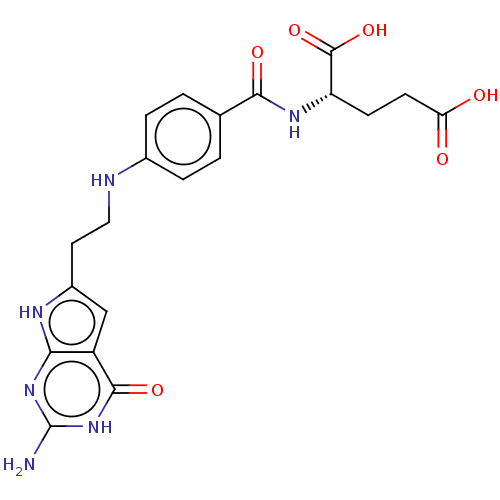 (CHEMBL4437824) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 60 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005518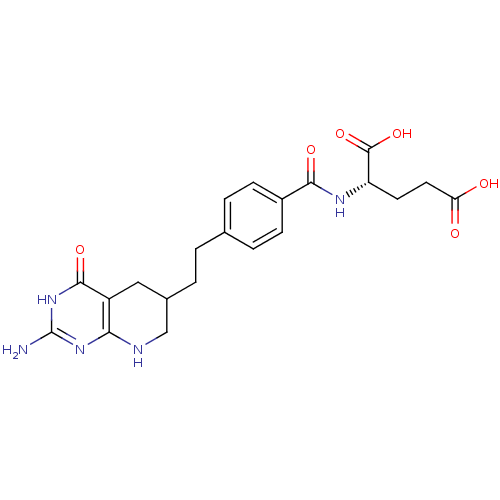 ((S)-2-(4-(2-((R)-2-amino-4-oxo-1,4,5,6,7,8-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant GAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 60 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534431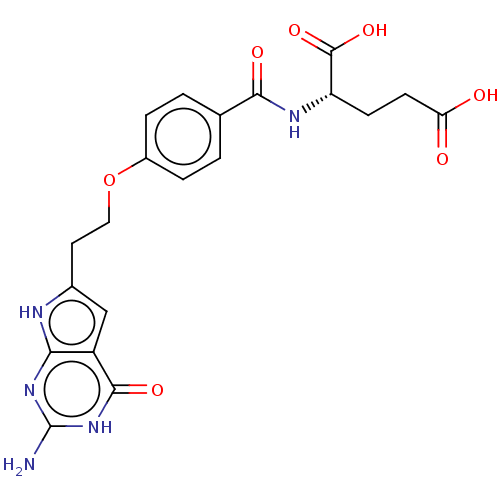 (CHEMBL4447805) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534429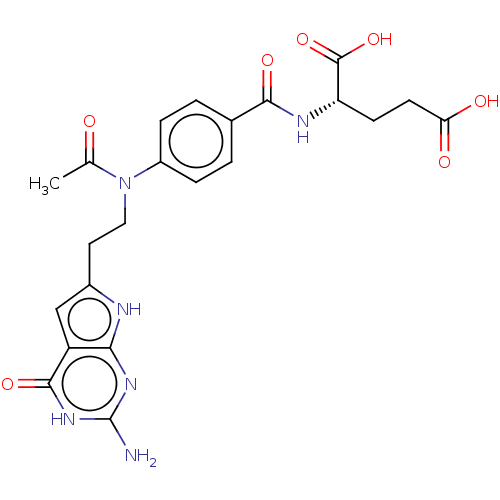 (CHEMBL4444011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50027656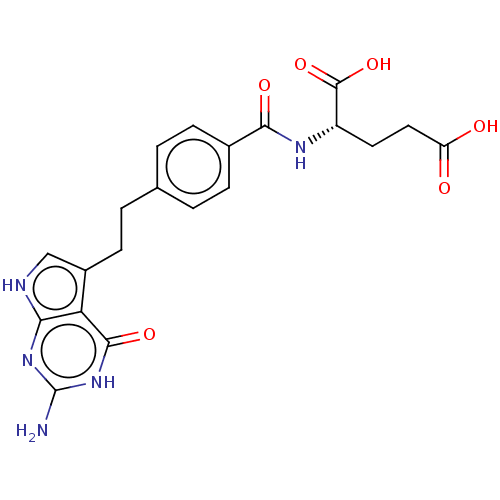 (CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50024475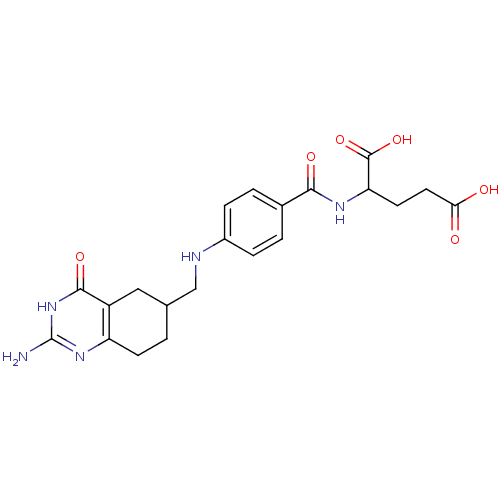 (2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse GAR transformylase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50354833 (AGF94 | CHEMBL1834488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,80-dideazafolic acid measured every 1... | J Med Chem 58: 6938-59 (2015) Article DOI: 10.1021/acs.jmedchem.5b00801 BindingDB Entry DOI: 10.7270/Q2QJ7K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50354833 (AGF94 | CHEMBL1834488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534430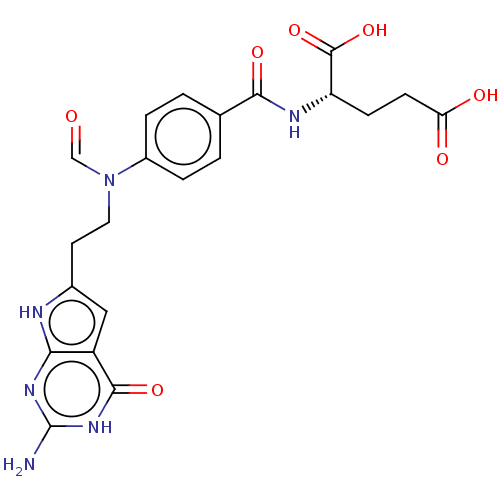 (CHEMBL4471269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50367875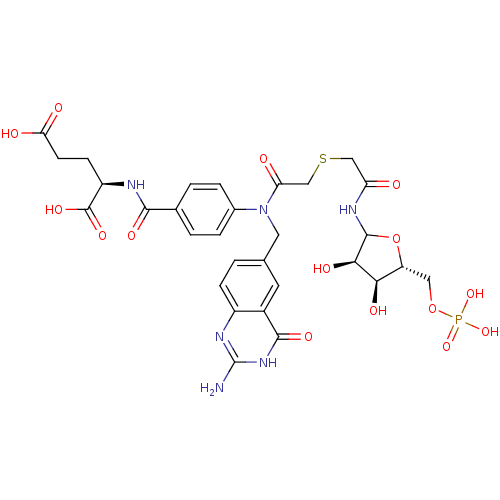 (CHEMBL607957) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Compound was tested for inhibition constant against GAR TFase in mice | J Med Chem 32: 937-40 (1989) BindingDB Entry DOI: 10.7270/Q2RX9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of trifunctional Glycinamide ribonucleotide formyltransferase isolated from murine L1210 cells. | Bioorg Med Chem Lett 3: 2657-2660 (1993) Article DOI: 10.1016/S0960-894X(01)80736-8 BindingDB Entry DOI: 10.7270/Q2R49QPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50003896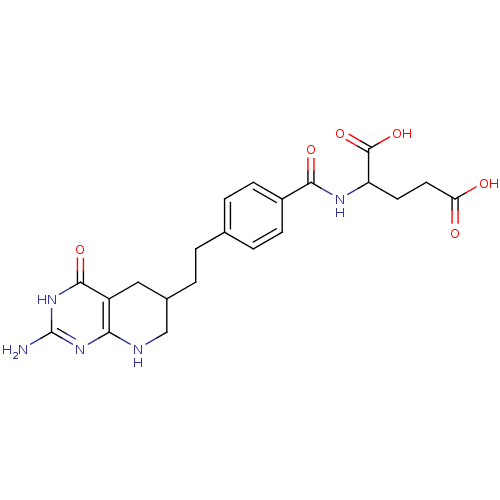 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534426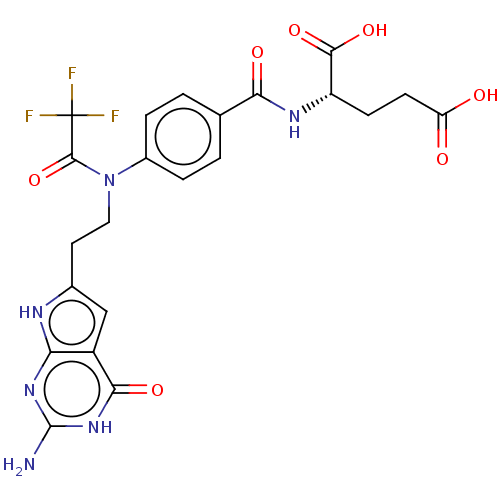 (CHEMBL4553188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of recombinant human monofunctional Glycinamide ribonucleotide formyltransferase | Bioorg Med Chem Lett 3: 2657-2660 (1993) Article DOI: 10.1016/S0960-894X(01)80736-8 BindingDB Entry DOI: 10.7270/Q2R49QPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50186739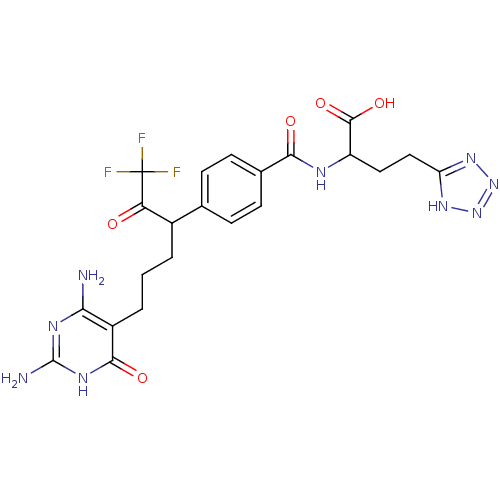 ((2S)-2-(4-(6-(2,4-diamino-6-oxo-1,6-dihydropyrimid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant GAR Tfase | J Med Chem 49: 2998-3002 (2006) Article DOI: 10.1021/jm0601147 BindingDB Entry DOI: 10.7270/Q2VX0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50291133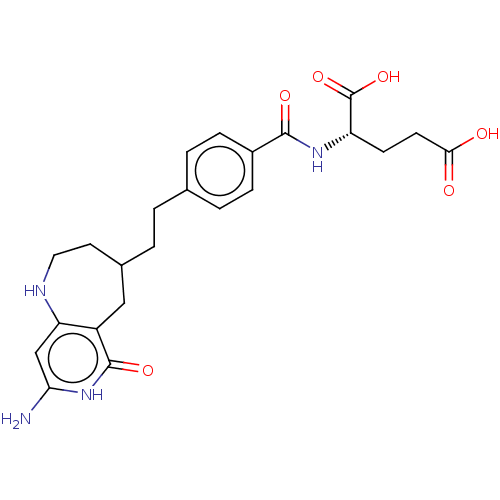 (2-{(S)-4-[2-(8-Amino-6-oxo-2,3,4,5,6,7-hexahydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50171513 ((S)-2-(4-(3-(2-amino-4-oxo-4,7-dihydro-3H-pyrrolo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24686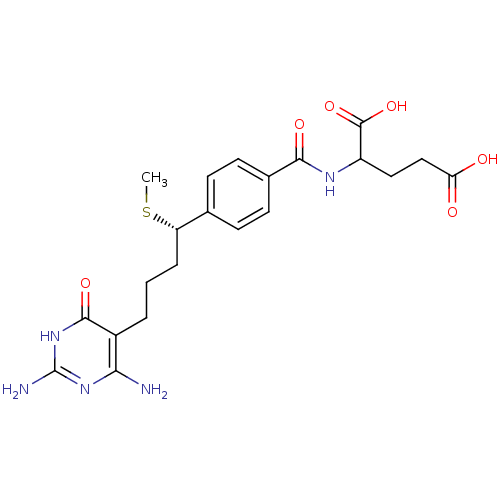 (10-thiomethyl-DDACTHF, 10S-3 | 10S (7) | 2-({4-[(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24685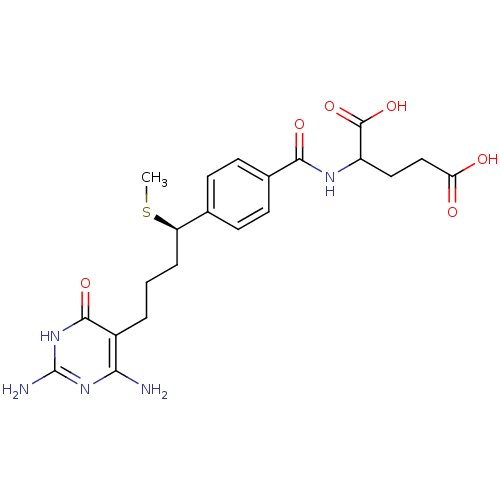 (10-thiomethyl-DDACTHF, 10R-3 | 10R (8) | 2-({4-[(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005865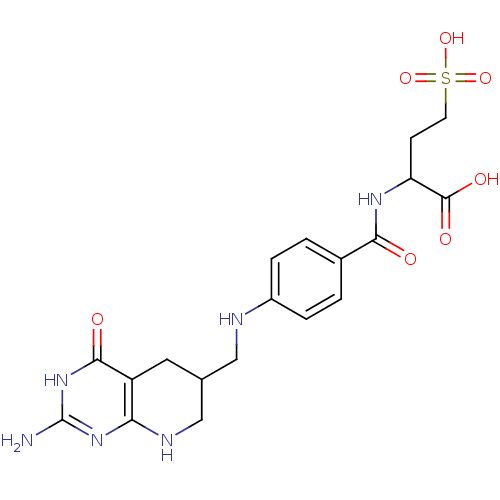 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50534428 (CHEMBL4467936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged human GARFTase assessed as formation of 5,8-dideazafolate from 10-formyl-5,8-dideazafolic acid measu... | J Med Chem 59: 7856-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00594 BindingDB Entry DOI: 10.7270/Q2NG4V5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24686 (10-thiomethyl-DDACTHF, 10S-3 | 10S (7) | 2-({4-[(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Enzyme activity assays of recombinant hGAR Tfase and recombinant hAICAR Tfase were performed as previously described. Kinetics of the enzyme reaction... | Biochemistry 52: 5133-44 (2013) Article DOI: 10.1021/bi4005182 BindingDB Entry DOI: 10.7270/Q2K93669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM24685 (10-thiomethyl-DDACTHF, 10R-3 | 10R (8) | 2-({4-[(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Med Chem 51: 5441-8 (2008) Article DOI: 10.1021/jm800555h BindingDB Entry DOI: 10.7270/Q2WD3XVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM18796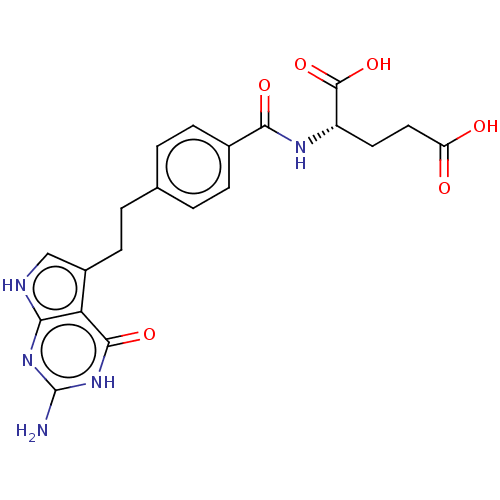 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH Curated by ChEMBL | Assay Description Inhibition of GARFT | J Med Chem 53: 6539-49 (2010) Article DOI: 10.1021/jm901869w BindingDB Entry DOI: 10.7270/Q24J0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50374378 (CHEMBL257691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human glycinamide ribonucleotide transformylase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 259 total ) | Next | Last >> |