

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26758 2-fluorophenyl N-[6-(2-methyl-4,5-diphenyl-1H-imidazol-1-yl)hexyl]carbamate::bisarylimidazole derivative, 17
SMILES: Cc1nc(c(-c2ccccc2)n1CCCCCCNC(=O)Oc1ccccc1F)-c1ccccc1
InChI Key: InChIKey=LHAHTHILLRCRBM-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26758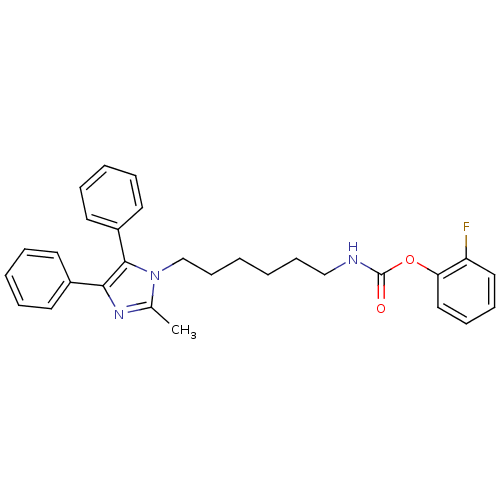 (2-fluorophenyl N-[6-(2-methyl-4,5-diphenyl-1H-imid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 9.0 | 22 |
Bristol-Myers Squibb Company | Assay Description FAAH Inhibition Assay [3H]Ethanolamine produced from [3H]AEA hydrolysis was used to calculate FAAH activity and was measured by scintillation countin... | Bioorg Med Chem Lett 17: 3287-91 (2007) Article DOI: 10.1016/j.bmcl.2007.04.009 BindingDB Entry DOI: 10.7270/Q2R20ZPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26758 (2-fluorophenyl N-[6-(2-methyl-4,5-diphenyl-1H-imid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of FAAH | J Med Chem 51: 7327-43 (2009) Article DOI: 10.1021/jm800311k BindingDB Entry DOI: 10.7270/Q2J67HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26758 (2-fluorophenyl N-[6-(2-methyl-4,5-diphenyl-1H-imid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in human H4 cells | Bioorg Med Chem Lett 20: 1272-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.080 BindingDB Entry DOI: 10.7270/Q2H41RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||